New measurements prove myosin VI can act as molecular transporter

In living organisms, hundreds of different kinds of molecular motors perform a variety of essential, but little understood tasks that result in such actions as muscle contraction, cell division and the movement of materials within cells. Some motors act as transporters, some serve as anchors, and some may do both.
New measurements performed at the University of Illinois at Urbana-Champaign have shown that one of these molecular motors, myosin VI, can function individually as anchors or in pairs as transporters. The new results have helped to resolve a research controversy and better explain how these little proteins perform their duties.
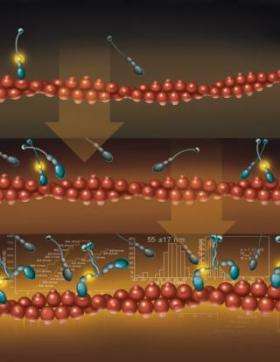
“Myosin VI is a tiny molecule that converts chemical energy into mechanical motion and transports its load by ‘stepping’ along filaments of actin,” said Paul Selvin, a John Bardeen Faculty Scholar of �鶹��Ժics at Illinois and a co-author of a paper to appear in the Feb. 3 issue of the journal Molecular Cell. “Our measurements prove that molecules of myosin VI can naturally form pairs and then go for walks while carrying cargo.”
In 2004, Selvin and colleagues at Illinois and at the University of Pennsylvania reported that myosin VI moved by a “hand-over-hand” mechanism in steps of approximately 60 nanometers..
“In that experiment, we took two myosin VI monomers and ‘zippered’ their tops together to form a dimer – which then walked along the actin,” Selvin said. “Some researchers thought that by forcing the molecules to dimerize, our results were not representative of what occurs naturally. Other researchers claimed that myosin VI was always a monomer. We have now shown that these molecules can indeed come together and dimerize on their own.”
In the latest experiment, the researchers repeated their earlier measurement, but without zippering the monomers together. They used a high-precision measurement technique developed at Illinois that can track the position of a molecule to within 1.5 nanometers. The technique is called FIONA (Fluorescence Imaging with One Nanometer Accuracy).
“We found that, at high enough concentrations, some of the myosin molecules would find one another, they would dimerize, and they would start walking,” Selvin said. “Those that did not dimerize remained monomers and did not move. We also found that when the cargo-binding domains were removed, the molecules dimerized more readily.
Selvin and his colleagues believe that myosin VI exists as a monomer if not bound to cargo. Binding to cargo alters the conformation of the molecule. At the same time, close proximity of two monomers bound to cargo initiates dimerization.
“It is possible that different cargos bind to different regions of the cargo-binding domain such that some cargos promote dimerization and others do not, allowing the protein to function as either a dimer (transporter) or a monomer (anchor), depending on its binding partners,” the researchers wrote in the Molecular Cell paper.
“And, while we have shown that myosin VI can be a transporter, we have not shown that it is a transporter,” Selvin said. “To do that, we have to look inside a living cell.”
Last year, Selvin’s team used FIONA to track the movements of two other molecular motors, dynein and kinesin, inside living fruit fly cells. “FIONA is capable of measuring the movement of myosin VI within living cells,” Selvin said. “We are working on that now.”
The co-authors of the paper are Selvin, Illinois graduate student and lead author Hyokeun Park; and Bhagavathi Ramamurthy, Mirko Travaglia, Dan Safer, Li-Qiong Chen, Clara Franzini-Armstrong and H. Lee Sweeney at the University of Pennsylvania.
Source: University of Illinois at Urbana-Champaign
















