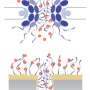
(Â鶹ŇůÔşOrg.com) -- To protect their DNA, cells in higher organisms are very choosy about what they allow in and out of their nuclei, where the genes reside. Guarding access is the job of transport machines called nuclear pore complexes, which stud the nuclear membrane. Despite these gatekeepers’ conspicuously large size (they are made of 30 different proteins), they have proved largely inscrutable to researchers over the years. But bit by bit, scientists are learning how these machines work.
Now a new study reveals the structure of one of the proteins that makes up this molecule-trafficking complex. Researchers have also shown how that protein interacts with a partner, supporting a model that calls for a flexible “ring” around the opening of each pore. The work could offer a key insight into an important design feature of this little-understood and evolutionarily ancient structure, an innovation fundamental to the development of nearly all multicellular life on Earth.
The research, performed by Hyuk-Soo Seo, a postdoctoral associate, and André Hoelz, a research associate, both in Rockefeller University’s Laboratory of Cell Biology, determined the molecular structure of the only remaining unsolved protein in an important piece of the nuclear pore called the Nup84 complex. Nup84 is a Y-shaped element that was recently imaged in three dimensions by Martin Kampmann, also a member of the lab headed by Howard Hughes Medical Institute Investigator Günter Blobel.
In experiments published online last week in the Proceedings of the National Academy of Sciences, Seo, Hoelz and colleagues focused on the behavior of this newly solved protein — or nucleoporin — called Nup120, one of seven comprising the Nup84 complex. They determined that one end of Nup120, the N-terminal domain, is attached by a stretchable tether to one other protein in the complex, Nup133. Furthermore, the researchers showed in living cells that mutations to a critical region of the tether interfered with the export of messenger RNA, one of the nuclear pore’s chief responsibilities, confirming the functional importance of this loose linkage between the two proteins.
“It’s a very nice correlation from the structure to the function,” Hoelz says. “It’s the first example where we can really pin down how the [Nup84] complexes arrange with each other, and what we believe we see is a flexible ring that could expand and contract to import and export large molecules.”
More information: Proceedings of the National Academy of Sciences online: August 11, 2009; ; Hyuk-Soo Seo, Yingli Ma, Erik W. Debler, Daniel Wacker, Stephan Kutik, Günter Blobel, and André Hoelz
Provided by Rockefeller University ( : )