Breakthrough in development of new drugs
A breakthrough made at STFC's ISIS neutron source could lead to faster and more cost-effective methods of developing new drugs.
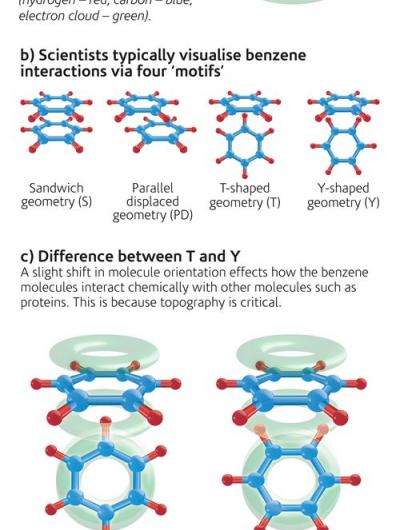
Scientists, led by University College London, have demonstrated how aromatic molecules arrange themselves in liquids, which turns out to be more complex than assumed when simulating these effects. Interactions between aromatic molecules - the most famous of which is benzene - are a factor in the way many important biological molecules including DNA and proteins are made up.
In the early stages of drug design, simulations are frequently used to determine the likely interaction of the drug with the target molecule. Current calculations are based on the interaction of just two aromatic molecules but new data from neutron experiments gives a more accurate picture of the way in which many molecules are orientated in the liquid and shows that this is an important factor in predicting their chemical reactions.
This discovery will have an impact ranging from basic chemistry through to our understanding of the self-assembly of biological molecules. It will in turn make drug discovery more efficient and accurate, reducing the time and cost of developing new drugs.
Provided by University College London
















