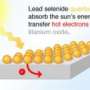
A dash of disorder yields a very efficient photocatalyst

(�鶹��ԺOrg.com) -- A little disorder goes a long way, especially when it comes to harnessing the sun’s energy. Scientists from the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) jumbled the atomic structure of the surface layer of titanium dioxide nanocrystals, creating a catalyst that is both long lasting and more efficient than all other materials in using the sun’s energy to extract hydrogen from water.
Their photocatalyst, which accelerates light-driven chemical reactions, is the first to combine durability and record-breaking efficiency, making it a contender for use in several clean-energy technologies.
It could offer a pollution-free way to produce hydrogen for use as an energy carrier in fuel cells. Fuel cells have been eyed as an alternative to combustion engines in vehicles. Molecular hydrogen, however, exists naturally on Earth only in very low concentrations. It must be extracted from feedstocks such as natural gas or water, an energy-intensive process that is one of the barriers to the widespread implementation of the technology.
“We are trying to find better ways to generate hydrogen from water using sunshine,” says Samuel Mao, a scientist in Berkeley Lab’s Environmental Energy Technologies Division who led the research. “In this work, we introduced disorder in titanium dioxide nanocrystals, which greatly improves its light absorption ability and efficiency in producing hydrogen from water.”
Mao is the corresponding author of a paper on this research that was published online Jan. 20, 2011 in Science Express with the title “Increasing Solar Absorption for Photocatalysis with Black, Hydrogenated Titanium Dioxide Nanocrystals.” Co-authoring the paper with Mao are fellow Berkeley Lab researchers Xiaobo Chen, Lei Liu, and Peter Yu.
Mao and his research group started with nanocrystals of titanium dioxide, which is a semiconductor material that is used as a photocatalyst to accelerate chemical reactions, such as harnessing energy from the sun to supply electrons that split water into oxygen and hydrogen. Although durable, titanium dioxide isn’t a very efficient photocatlayst. Scientists have worked to increase its efficiency by adding impurities and making other modifications.
The Berkeley Lab scientists tried a new approach. In addition to adding impurities, they engineered disorder into the ordinarily perfect atom-by-atom lattice structure of the surface layer of titanium dioxide nanocrystals. This disorder was introduced via hydrogenation.
The result is the first disorder-engineered nanocrystal. One transformation was obvious: the usually white titanium dioxide nanocrystals turned black, a sign that engineered disorder yielded infrared absorption.
The scientists also surmised disorder boosted the photocatalyst’s performance. To find out if their hunch was correct, they immersed disorder-engineered nanocrystals in water and exposed them to simulated sunlight. They found that 24 percent of the sunlight absorbed by the photocatalyst was converted into hydrogen, a production rate that is about 100 times greater than the yields of most semiconductor photocatalysts.
In addition, their photocatalyst did not show any signs of degradation during a 22-day testing period, meaning it is potentially durable enough for real-world use.
Its landmark efficiency stems largely from the photocatalyst’s ability to absorb infrared light, making it the first titanium dioxide photocatalyst to absorb light in this wavelength. It also absorbs visible and ultraviolet light. In contrast, most titanium dioxide photocatalysts only absorbs ultraviolet light, and those containing defects may absorb visible light. Ultraviolet light accounts for less than ten percent of solar energy.
“The more energy from the sun that can be absorbed by a photocatalyst, the more electrons can be supplied to a chemical reaction, which makes black titanium dioxide a very attractive material,” says Mao, who is also an adjunct engineering professor in the University of California at Berkeley.
The team’s intriguing experimental findings were further elucidated by theoretical physicists Peter Yu and Lei Liu, who explored how jumbling the latticework of atoms on the nanocrystal’s surface via hydrogenation changes its electronic properties. Their calculations revealed that disorder, in the form of lattice defects and hydrogen, makes it possible for incoming photons to excite electrons, which then jump across a gap where no electron states can exist. Once across this gap, the electrons are free to energize the chemical reaction that splits water into hydrogen and oxygen.
“By introducing a specific kind of disorder, mid-gap electronic states are created accompanied by a reduced band gap,” says Yu, who is also a professor in the University of California at Berkeley’s �鶹��Ժics Department. “This makes it possible for the infrared part of the solar spectrum to be absorbed and contribute to the photocatalysis.”
This research was supported by the Department of Energy’s Office of Energy Efficiency and Renewable Energy. Transmission electron microscopy imaging used to study the nanocrystals at the atomic scale was performed at the National Center for Electron Microscopy, a national user facility located at Berkeley Lab.
Provided by Lawrence Berkeley National Laboratory