New clues for asthma treatment
(�鶹��ԺOrg.com) -- New information that could help in the fight against asthma has been obtained by an international collaboration of scientists utilizing the U.S. Department of Energy’s Advanced Photon Source at Argonne National Laboratory. Their results, which were recently published in the journal Nature, show how an important human transmembrane protein functions at a molecular level. The findings are significant in that the particular human transmembrane protein known as β2-adrenergic receptor, a G protein-coupled receptor (GPCR), is the focus of a series of drugs for the treatment of asthma. This new research on its structure and function has the potential of leading to the development of improved drug therapies.
There are over 750 human GPCRs distributed throughout the body with representatives in almost every cell type. They function in myriad ways enabling us to interact with our environment, and with one another through the sense of sight and smell. They also play key roles in heart and lung function, in how we respond to hormones and neurotransmitters and by extension how they influence mood and behavior, and are involved in immunity and inflammation. It is apparent, therefore, why a full suite of properly functioning GPCRs is integral to human health and wellbeing. About a third of drugs on the market today target GPCRs.
The research, by investigators from the Stanford University School of Medicine, Trinity College Dublin, the University of Limerick, Friedrich Alexander University, D. E. Shaw Research, the University of Michigan Medical School, and the University of Wisconsin-Madison set out to understand how one of these GPCRs, the β2-adrenergic receptor, works at a molecular level. The receptor is long enough to comfortably span the 5-nanometer width of the cell’s outer protective membrane. In this way, one end of the receptor can sense what is happening outside the cell and transmit the information it collects there to the cell’s interior for appropriate action. The fight-or-flight hormone adrenaline mediates its activity by way of the β2-adrenergic receptor. When the receptor binds adrenaline shot into the blood by the adrenal gland it snaps into action by binding with its cognate G-protein located inside the cell. This interaction triggers a series of reactions within and between cells that are part of the body’s response to adrenaline. These include vasodilation and constriction, smooth muscle relaxation, hearth muscle contraction, and mobilization of energy reserves in the liver and muscle. The β2-adrenergic receptor is an important pharmaceutical paradigm for the larger family of GPCRs, some of which are targeted by beta blockers.
Martin Caffrey, Trinity College Professor of Membrane Structural and Functional Biology in the Schools of Medicine and Biochemistry & Immunology explained: “New and improved drugs are always in demand. But to design them in a rational way we need to know how the receptor, in this case the β2-adrenergic receptor, is put together in a structural sense and how this structure enables it to function as a receptor and a communicator of information.
“By structure we refer to the arrangement in three-dimensions of the receptor’s constituent atoms, amino acids, and the ligands it binds. We would also like to know how this structure changes when adrenaline nudges the receptor and how this facilitates downstream signaling.
“The only way to get such detailed information for a complicated membrane protein like the β2-adrenergic receptor is to use macromolecular crystallography, which requires a well-ordered crystal of the receptor. The crystals must then be irradiated with x-ray photons in a way that can be used to decipher the receptor’s structure.
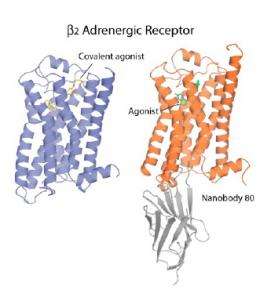
“One of the big challenges in this protracted and involved process is to coax the protein into the regular and ordered lattice of a crystal,” said Caffrey. “This is particularly difficult in the case of GPCRs because they continually flit about structurally in the plane of the membrane and, at any one moment, can be seen to exist in a number of different conformations or shapes. The particular conformation assumed, in turn, dictates the receptor’s biological activity. To get a collection of receptor molecules to crystallize, ideally they should all assume the one stance or conformation.”
Brian Kobilka, Professor of Molecular and Cellular �鶹��Ժiology at Stanford University, who led this research project, devised a strategy for locking the receptors into what amounts to a single conformation by covalently or irreversibly splicing an adrenaline look-alike molecule into the binding site of the receptor. So stabilized and rendered uniform, the receptor was successfully crystallized and its structure solved.
The study featured the use of a novel, high-throughput method and instrumentation, developed by the Trinity team members that crystallized the stabilized β2-adrenergic receptor. Custom-designed robots dispensed nanoliter volumes of a highly viscous, protein-laden lipidic liquid crystal or mesophase into home-built, multi-well glass sandwich plates for crystallization screening. The mesophase mimics the lipid bilayer membrane in which the receptor resides in the cell. When treated appropriately it undergoes a transition to a second mesophase in which receptor molecules preferentially cluster, arrange themselves regularly in two- and then three-dimensional arrays, and eventually form crystals. The crystals typically are very small, just a tenth to a hundredth of a millimeter in size, and are extremely fragile. They were harvested carefully from the toothpaste-textured mesophase, cryo-cooled in liquid nitrogen, and then shipped in special dewars from the laboratory at Trinity to the General Medicine and Cancer Institutes Collaborative Access Team facility on x-ray beamline 23-ID at the Advanced Photon Source. There, the team used state-of-the-art technologies to center the crystal in the x-ray beam and collect diffraction data, which, upon processing and modeling by researchers at Stanford, generated the structure reported in Nature.
More information: Daniel M. Rosenbaum, et al., “Structure and function of an irreversible agonist-β2 adrenoceptor complex,” Nature 469, 236 (13 January 2011)
Provided by Argonne National Laboratory















