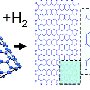September 15, 2011 feature
Inside story: Chemical reactivity on the inner surface of single-walled carbon nanotubes
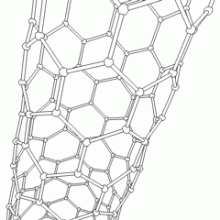
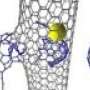
(�鶹��ԺOrg.com) -- Historically, the interior surface of single-walled carbon nanotubes (SWNTs) has not been considered to be chemically reactive. Recently, however, researchers at the in the UK and the in Germany demonstrated sidewall (inner surface) chemical reactions when they inserted catalytically active atoms of rhenium metal (Re) into these atomically thin cylinders of carbon. These reactions formed nanometer-sized hollow protrusions in three distinct phases (sidewall deformation and rupture, open nanoprotrusion formation, and stable closed nanoprotrusion) which the researchers imaged at the atomic level – in real time at room temperature – using Aberration-Corrected High-Resolution Transmission Electron Microscopy (AC-HRTEM).
Prof. Andrei N. Khlobystov conceived of the initial idea, proposed the general mechanism and wrote the original manuscript; Thomas W. Chamberlain designed the experiments, synthesized the materials and analyzed the microscopy data; Ute Kaiser contributed to the development of the experimental methodology and discussion of the results; Elena Bichoutskaia, Nicholas A. Besley and Adriano Santana performed the theoretical modeling and explained the details of the reaction mechanisms; and Johannes Biskupek analyzed the images, carried out TEM image simulations, and – with Jannik C. Meyer and Jens Leschner – recorded the AC-HRTEM images and contributed to the initial explanation of the observations.
The main experimental challenge the team faced was to devise a method for delivering single atoms of catalytically active metal into very narrow carbon nanotubes with a diameter of 1.5 nm – about 80,000 times smaller than the thickness of human hair. “The presence of such metal atoms within the nanotube is important not just for investigating the chemical reactivity of the inner sidewall, but also for creating new nanostructures from the nanotube,” notes Khlobystov.
The second major challenge, he adds, “was to study the delicate molecules, reactive atoms and their chemical transformation inside nanotubes in real-time at the atomic level.”
To address these challenges, the team exploited the remarkable affinity of carbon nanotube with fullerenes – carbon nanostructures, which look like nanometer-sized cages and can be considered as structurally related to nanotubes. “The fullerenes are known to be attracted strongly into the nanotube cavity by van der Waals forces. We tagged each fullerene with a single atom of rhenium metal, so that each molecule brings a catalytically active metal atom into the nanotube,” Khlobystov explains. “It appears that such modified fullerenes are excellent vehicles for delivery of metal atoms into nanotubes, as they enter in nanotube spontaneously and irreversibly.”
The second challenge, he continues, was solved by the researchers in Ulm, who applied a specially designed electron microscope that utilizes low energy electrons for imaging molecules and atoms. “They have succeeded at imaging the delicate molecules with atomic resolution and, most importantly, at capturing them in action – i.e., in chemical processes within the carbon nanotube in real time.”
Kaiser comments that “Our aim is use low voltage TEM – which is now possible after the introduction of hardware aberration correction by Harald Rose, Max Haider and Knut Urban – to study in detail the atom-by-atom level influence of electron-beam interacting with low-Z matter,” which is matter with a low atomic number. “To accomplish this we developed the real-time imaging and data acquisition technology to reveal carbon nanotubes and their interior in high contrast and atomic resolution.
“In order to provide a comprehensive description of a possible mechanism for nanoprotrusion formation on carbon nanotube walls," adds Bichoutskaia, “we used a multi-scale modeling approach that combined accurate quantum chemical methods with semi-empirical molecular dynamics simulations.”
Going forward, there are a number of innovations that might be developed and applied to the current experimental design – for example, catalysts other than rhenium, carbon sources other than the fullerene cage wall, nanotubes produced or grown using an alternative method, nanotubes using different fullerene, or variations in the e-beam. “Our next steps include implementing catalysts and more complex molecules into carbon nanotubes,” Kaiser confirms. “We’re also working on varying the e-beam energy and detection efficiency in our Sub-Angström Low-Voltage Electron (SALVE) microscopy project at Ulm University.”
Khlobystov points out that there are dozens of different metals in the Periodic Table of elements, and each of them has a distinct set of useful physicochemical properties that could be harnessed at the single-atom level. “Our method of transport and encapsulation of metals into nanotubes is quite universal, as it can be adapted for any of the transition metals, many of which have outstanding chemical, optical and magnetic properties,” he explains. “For example, introduction of photoactive atoms into carbon nanotubes, such as ruthenium or platinum, may enable initiation and control of chemical reactions within nanotubes using pulses of light, which would be more useful than an electron beam for practical applications.”
Furthermore, transition metals with well-defined catalytic properties different from those of rhenium, such as palladium, platinum, rhodium, and nickel, could trigger entirely different reactions in nanotubes, leading to different products that are difficult to anticipate at this stage – but Khlobystov is confident that within the next 12 months the team will be able to tell exactly what can be achieved with other types of metals. “Even now,” he stresses, “we know that addition of non-metallic elements other, such as sulfur, into nanotubes can drastically change the course of chemical reactions inside the nanotube.” Recently, the team published a paper showing that when sulfur and carbon are present in nanotube together, we can form unique nanoribbon structures with remarkable properties.”
In terms of how their research might impact the design and/or development of electronic, medical, sensor or other nanoscale devices, Khlobystov notes that since carbon nanotubes are ideal containers for molecules and atoms, “With one macroscopic dimension,” being length, “and two nanoscopic dimensions, they can serve as a bridge between the molecular and the macroscopic worlds. Magnetically active molecules embedded in nanotubes, for example, could be integrated in miniature data storage and spintronic devices, and nanotubes could be used as a capsule for delivery of medicinal molecules directly into diseased cells in human body.” Moreover, Khlobystov notes that the electronic properties of the nanotube itself, such as band gap and charge carrier concentrations and mobility, are greatly affected by interactions with the guest molecules inside the nanotube, which forms a basis for sensors and detectors.
“Furthermore,” he adds, “development of nanotubes as chemical reactors is a very promising direction, as pathways and rates of chemical reactions confined in nanotubes are drastically affected by the nanotube. Chemical synthesis in nanotubes is a new way of making molecules that will enable us to make new products that are not possible to prepare otherwise. Catalysis by transition metals is essential in this context, and understanding direct reactions of metals with nanotubes is the first step.”
Kaiser believes that besides chemists and physicists working on basic research, nanotechnologists devoted to topics such as energy storage, catalysis and medical drug delivery both on hard-, soft- and combined hard-soft matter will benefit from the team’s research. “New technologies in TEM control, efficiency that allows us to detect every scattered electron, and goniometer design that is not disturbed by drift issues during TEM data acquisition will strongly enhance the new applications.” (A goniometer allows a specimen to be rotated to a precise angular position.)
Kaiser agrees that carbon nanotube spontaneous self-assembly and interior nanoprotrusion formation, which all may open new avenues for nanoscale molecular synthesis. She also cites the effect of confinement within carbon nanotubes as well as the newly shaped CNT with nanoprotrusions as potentially providing a new mechanism for tuning the electronic properties of graphene nanoribbons. “The spectacular rotational and translational motion of helical nanoribbons within the nanotube, she adds, “as well as the possible regular formation of nanoprotrusions may inspire the exploration and harnessing of new electromechanical effects in nanodevices.”
In the short-term, Khlobystov points out, the team is rapidly expanding the range of transition metals inserted into nanotubes to broaden the scope of chemical reactions studied under conditions of extreme confinement and, at the same time, to see whether the nanotube sidewall could be engaged in further, perhaps even more spectacular chemical transformations. “So far, our experiments have been carried out on a small scale, so our process would also need to be scaled up to test and explore real applications of these materials,” he acknowledges.
For Kaiser, next steps include imaging more complex structures at the present 80kV aberration-corrected TEM and at 20kV with our new SALVE prototype microscope. “We will explore the electron-beam specimen interaction further and will probably discover further surprises,” she adds.
The potential for an in vivo application remains uncertain. “At the moment,” Khlobystov opines, “I can’t really see how our process can be transferred to an in vivo protocol. The conditions required to spark chemical transformations in nanotubes are still very harsh. However, if a living system would possess some sort of super-enzyme that is able to crack carbon-carbon bonds of the nanotube sidewall, in principle, we could adopt our nanoreactors for a biological system.”
Kaiser admits that this is rather speculative, noting the additional limitation that in vivo atomic resolution is not obtainable today. “However,” she opines, “with our SALVE initiative a new low-voltage TEM will be finalized in two years through our collaborations with partners CEOS and Carl Zeiss, we will be a step closer to image beam-sensitive biological materials.”
Khlobystov emphasizes that these exciting applications rely on a well-defined and reliable interface between the nanotube container and the contained molecules and atoms. “Because a pristine nanotube has an atomically smooth surface, the molecules shuttle randomly from one position to another within the nanotube in almost frictionless motion. Nanoprotrusions formed on nanotubes in our experiments create hollow pockets on the nanotube inner surface, which can effectively trap desired molecules and atoms in a specific location, thus giving a mechanism for controlling their positions and orientations. A greater degree of control over the dynamic behavior of encapsulated molecules is essential,” he concludes, “for successfully harnessing the full potential of their optical, magnetic and chemical properties.”
More information: Reactions of the inner surface of carbon nanotubes and nanoprotrusion processes imaged at the atomic scale, Nature Chemistry (2011), , Published online 14 August 2011
Related: Self-assembly of a sulfur-terminated graphene nanoribbon within a single-walled carbon nanotube, Nature Materials 10, 687–692 (2011), , Published online 07 August 2011
Copyright 2011 �鶹��ԺOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of �鶹��ԺOrg.com.