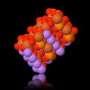
Transmission electron microscopy image of carbon nitride created by the reaction of carbon dioxide and Li3N.
(�鶹��Ժ) -- A materials scientist at Michigan Technological University has discovered a chemical reaction that not only eats up the greenhouse gas carbon dioxide, it also creates something useful. And, by the way, it releases energy.
Making carbon-based products from CO2 is nothing new, but carbon dioxide molecules are so stable that those reactions usually take up a lot of energy. If that energy were to come from fossil fuels, over time the chemical reactions would ultimately result in more carbon dioxide entering the atmosphere—defeating the purpose of a process that could otherwise help mitigate climate change.
Professor Yun Hang Hu’s research team developed a heat-releasing reaction between carbon dioxide and Li3N that forms two chemicals: amorphous carbon nitride (C3N4), a semiconductor; and lithium cyanamide (Li2CN2), a precursor to fertilizers.
“The reaction converts CO2 to a solid material,” said Hu. “That would be good even if it weren’t useful, but it is.”
And how much energy does it release? Plenty. Hu’s team added carbon dioxide to less than a gram of Li3N at 330 degrees Celsius, and the surrounding temperature jumped almost immediately to about 1,000 degrees Celsius, or 1,832 degrees Fahrenheit, about the temperature of lava exiting a volcano.
Hu’s work is funded by the National Science Foundation and detailed in the article authored by Hu and graduate student Yan Huo and published in the Journal of �鶹��Ժical Chemistry.
Provided by Michigan Technological University