Elusive atmospheric compound revealed in the laboratory

(Â鶹ÒùÔº)—Like a talented escape artist, this atmospheric performer has managed to hide its modus operandi—until now. Scientists at Pacific Northwest National Laboratory and the University of California-San Diego have exposed the antics of an organic or carbon-containing compound and how it reacts with water in the atmosphere to complete its escape act. Documenting the particle's MO gives scientists a way to track this atmospheric player and has implications for understanding its warming and cooling effects on the climate. Their research was recently published in Aerosol Science and Technology.
There are carbon-containing compounds in the atmosphere, from both human-caused emission sources and from plant-based emissions. They are suspended in the atmosphere, along with a veritable aerosol soup of water, gases, dust, and soot, and are continuously reacting to form other compounds. Because many of these ingredients influence the way sunlight scatters or is absorbed in the atmosphere, scientists are working to single out these constituents to find the role each plays in the warming and cooling of the climate.
Organonitrates, compounds formed from volatile organic compounds in the presence of nitrogen oxides (NOx), have long been predicted to be abundantly present in the atmosphere. But direct observations to date had not confirmed that prediction. Two causes are suspect in missing their presence: the chief instrument used for atmospheric sampling is not sensitive to their presence and researchers suspected that these compounds react so quickly with water to form other compounds that they are not detected.
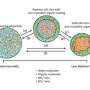
The research team used the Environmental Chamber located at PNNL in the Atmospheric Measurements Laboratory to observe the reaction of these molecules. They injected gas-phase organic precursor compounds into the chamber, then started photooxidation reactions by turning on the chamber's UV lights. Reacting in the presence of light and NOx, the organic gases oxidize forming a myriad of products, including organic nitrates, some of which condense to form particles. The team then measured the presence of organonitrates in the particles under different relative humidity conditions and measured how fast they react with water.
The study concluded that these constituents are efficiently generated in the gas phase and condense to the particle phase in significant quantities. In the particle phase, they are initially light-absorbing, but as they react with water that ability quickly wanes. The laboratory observations in the chamber enabled the team to gain insights on how the particles initially absorb light, thus having a warming effect on the climate, then have a cooling effect as they age. The measurements also help researchers interpret field observations.
This research has fit another piece into the atmospheric puzzle. The team's progress opens the door for further collaboration by examining atmospheric organic particles using laboratory recreation of atmospheric processes.
More information: Liu S, JE Shilling, C Song, N Hiranuma, RA Zaveri, and LM Russell. 2012. "Hydrolysis of Organonitrate Functional Groups in Aerosol Particles." Aerosol Science and Technology 46(12): 1359-1369.
Provided by Pacific Northwest National Laboratory