Room temperature oxidation of carbon monoxide with 100% efficiency
Ultra-thin 1-dimensional (1D) nanowires (NWs) have emerged as a new class of effective nanoscale catalysts, exhibiting impressive activity and durability, as demonstrated by their excellent performance in fuel cell reactions.
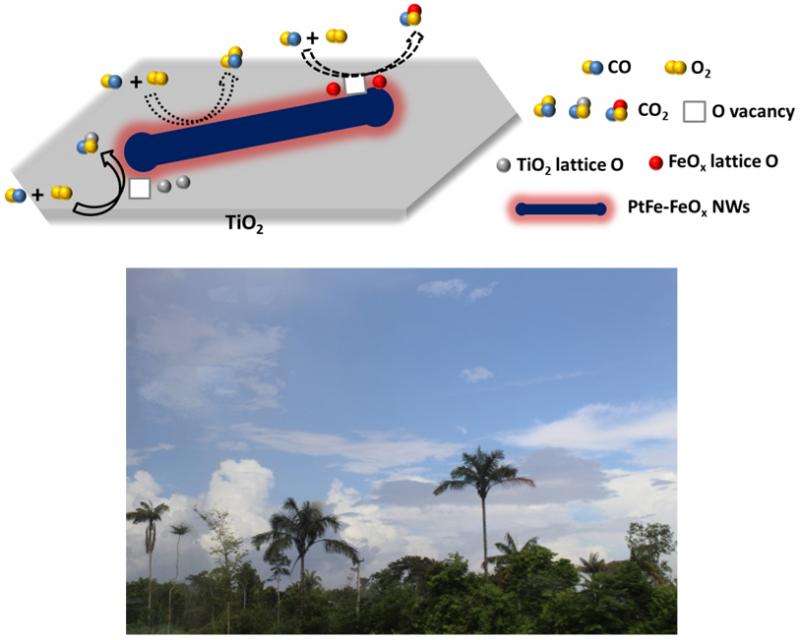
A collaborative effort among scientists from Oak Ridge National Lab, the University of Tennessee, Zhejiang University of Technology, and CFN has produced unique 1D, core-shell PtFe-FeOx NWs, supported on a TiO2 substrate, that yield highly efficient, room temperature carbon monoxide (CO) oxidation. Atomic level interactions between the PtFe core and the FeOx shell and interactions between the NWs and the TiO2 substrate enabled CO oxidation with 100% conversion efficiency at room temperature. Additionally, after 30 hours in the reaction environment, the PtFe−FeOx/TiO2 NW catalyst exhibited no decay in the catalytic activity. These results provide a general approach and new insights into the construction of hierarchical interfaces for advanced catalysis. These results also could provide a highly efficient and potentially cost-effective means for carbon monoxide air pollution abatement and CO-based fuel cell operation.
These 1D nanostructures enable CO oxidation with 100% conversion efficiency at room temperature, which could provide insight into the production of hierarchical interfaces for advanced catalysis. Further, this work could eventually lead to a highly efficient and cost-effective means for CO air pollution abatement.
The CFN Electron Microscopy Facility's HD2700C Scanning Transmission Electron Microscope was used to characterize the samples.
More information: "Constructing Hierarchical Interfaces: TiO2-Supported PtFe–FeOx Nanowires for Room Temperature CO Oxidation." J. Am. Chem. Soc., 2015, 137 (32), pp 10156–10159
Provided by Brookhaven National Laboratory