Self-assembling material that grows and changes shape could lead to artificial arteries
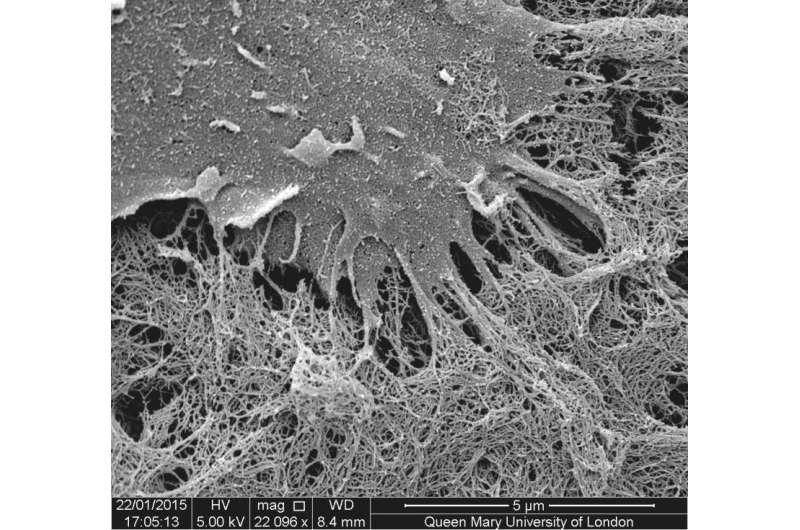
Researchers at Queen Mary University of London (QMUL) have developed a way of assembling organic molecules into complex tubular tissue-like structures without the use of moulds or techniques like 3D printing.
The study, which will appear on Monday 28 September in the journal Nature Chemistry, describes how peptides and proteins can be used to create materials that exhibit dynamic behaviors found in biological tissues like growth, morphogenesis, and healing.
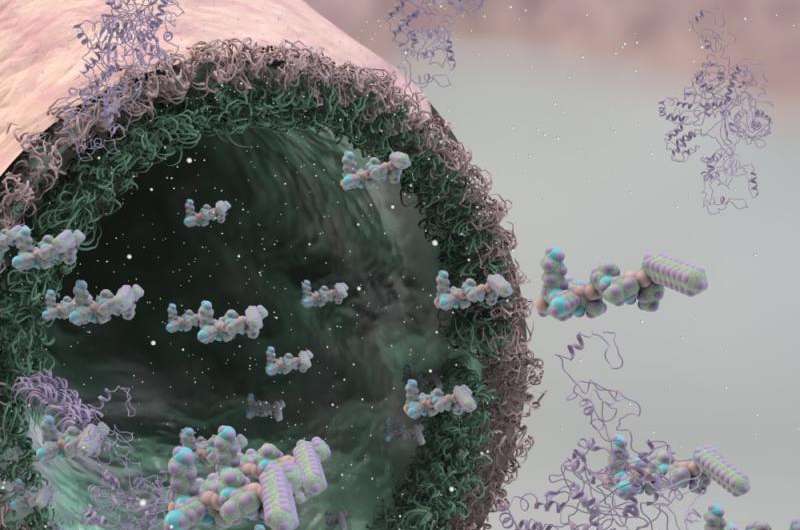
The method uses solutions of peptide and protein molecules that, upon touching each other, self-assemble to form a dynamic tissue at the point at which they meet. As the material assembles itself it can be easily guided to grow into complex shapes.
This discovery could lead to the engineering of tissues like veins, arteries, or even the blood-brain barrier, which would allow scientists to study diseases such as Alzheimer's with a high level of similarity to the real tissue, which is currently impossible. The technique could also contribute to the creation of better implants, complex tissues, or more effective drug screening methods.
Alvaro Mata, Director of the Institute of Bioengineering at QMUL and lead author of the paper, said:"What is most exciting about this discovery is the possibility for us to use peptides and proteins as building-blocks of materials with the capacity to controllably grow or change shape, solely by self-assembly.
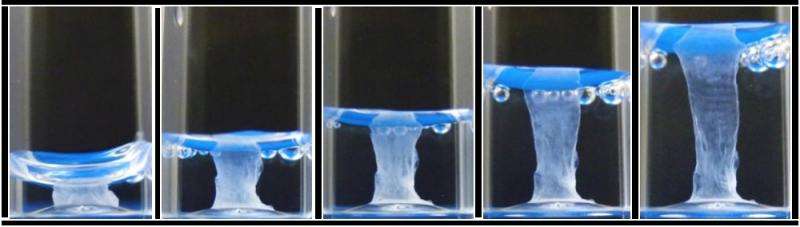
Karla Inostroza-Brito, PhD student and first author of the paper said: "The system is dynamic so it can be triggered on demand to enable self-assembly with a high degree of control, which allows the creation of complex shapes with a structure that resembles elements of native tissue."
Endorsing the research, Professor Dietmar W. Hutmacher, from Queensland University of Technology, said: "The quest for the 21 century tissue engineering concepts is not to replicate/copy the physical structure of the tissue to be regenerated but to guide the regeneration process by providing a scaffold that inherits a sculupturous structural design combined with biomolecules which give the crucial signals for guiding the regeneration. The discoveries presented in the paper by Alvaro Mata's group in Nature Chemistry are built on this new paradigm. The results are eminent to usher the next era of design and fabrication of scaffolds for tissue engineering applications."
More information: Co-assembly, spatiotemporal control and morphogenesis of a hybrid protein–peptide system,
Journal information: Nature Chemistry
Provided by Queen Mary, University of London

















