The main authors of the study (from left): Molecular biologist Dr. Sebastian Leidel, biochemist Katja Hartstock (lead author), molecular biologist Benedikt Nilges und biochemist Professor Andrea Rentmeister. Credit: WWU/E. Wibberg
What happens in a cell when genetic information is translated into proteins? In order to study this process, researchers study one particular biomolecule inside the cell: messenger ribonucleic acid, mRNA for short. This biomolecule plays a major role in all cellular processes—and it is also the focus of joint research being carried out by two groups at the Cells-in-Motion Cluster of Excellence at Münster University. One of the groups consists of biochemists and is headed by Prof. Andrea Rentmeister; the other is made up of molecular biologists and is led by Dr. Sebastian Leidel.
In their interdisciplinary collaboration, the researchers have succeeded for the first time in chemo-enzymatically labeling an important change in messenger RNA—the so-called m6A modification—and subsequently detecting it by means of modern molecular biological methods. "This new approach enables us to locate modifications in mRNA with a greater degree of accuracy than ever before," says Andrea Rentmeister, a professor at the Cluster of Excellence who led the study. Knowing where and to what extent m6A modifications occur could help researchers to understand this modification in physiological and pathological processes. The study has been published in the Angewandte Chemie (International Edition) journal.
The genetic information of the DNA is transcribed into messenger RNA in a process known as transcription. Following transcription, mRNA transports the genetic information from the cell nucleus into the cytoplasm. There, it serves as a guide for the production of proteins. Proteins are the workhorses that carry out all the cellular tasks.
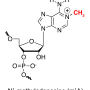
Like double-stranded DNA, single-stranded RNA consists of a chain of so-called nucleotides. In RNA, however, there are also many chemical changes to these nucleotides—known as RNA modifications. These modifications occur after the genetic information has been read. In the process, simple atomic arrangements—the methyl groups—are attached to the nucleotides. "One modification currently being hotly debated is the N6-methyladenosine, known as m6A for short," says Andrea Rentmeister. This modification is highly interesting because it appears to be responsible for a series of biological processes including the circadian clock. It also seems to play a role in pathological processes, for example in some forms of cancer or in viral infections.
Biochemists labelled RNA modifications chemo-enzymatically
In order to gain a better understanding of m6A, the researchers sought to find where exactly in the mRNA the modification located. To label molecules, biologists often use antibodies that attach themselves to it. This method has its limitations, however; the antibodies can bind not only to the modifications of the mRNA, but also to neighbouring nucleotides. This makes it difficult to locate the modifications precisely. "We now wanted to carry out the labeling with a chemical approach," Andrea Rentmeister explains. For the first time, she and her team used propargyl groups, a slightly longer hydrocarbon residue.
The researchers coupled the propargyl groups to the cosubstrate of the enzyme, and combined all three components with mRNA molecules in the test tube. In its chemical structure, propargyl is similar to a natural molecule bound by a methyltransferase. Methyltransferases for their part are enzymes that are responsible for the modification of mRNA. Thus, the methyltransferases were able to transfer the propargyl group to the RNA. Using so-called click chemistry, the scientists were able to isolate and purify the RNA with propargyl groups.
Molecular biologists detected RNA modifications using Next Generation Sequencing
In order to detect the specifically labelled modifications, the researchers used a special enzyme to transcribe mRNA back into DNA. The resulting DNA strand is a copy of the previous RNA and can be investigated using molecular biological methods. The researchers sequenced this newly synthesized DNA strand, reading the sequences of nucleotides. They used next-generation sequencing, which enabled them to determine the sequences of nucleotides extremely efficiently. "This method allows us to analyse thousands of sequences in parallel," explains Sebastian Leidel.
Because the researchers had labeled the modifications with the propargyl groups, the enzymes necessary for the rewriting of the RNA arrested. As a result, they failed to transcribe the RNA back into DNA. "The enzymes ceased any activity at the labeled sites and generated some kind of stop signal," says Katja Hartstock, a chemist and lead author of the story. The researchers were able to determine these stop signals during the sequencing, which meant that they could detect the sites at which the mRNA modification occurred.
After the initial experiments in the test tube, the researchers applied their new method in a culture of human epithelial cells—HeLa cells. The researchers fed the cells with a propargyl-labelled so-called amino acid precursor, which the cells "ate," and subsequently started the labeling. As already established in the test tube, the propargyl groups attached themselves to the RNA with the help of methyltransferases and allowed the detection of the mRNA modification sites by Next generation sequencing.
The next step the researchers want to take is to apply their method to living organisms in order to study the significance of the modification within their development. Zebrafish are well suited for this purpose as they develop very fast and the modifications are therefore transcribed faster—and are also removed again faster.
More information: Katja Hartstock et al, Enzymatic or In Vivo Installation of Propargyl Groups in Combination with Click Chemistry for the Enrichment and Detection of Methyltransferase Target Sites in RNA, Angewandte Chemie International Edition (2018).
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by University of Münster