Mind your Qs: PolyQ-binding protein 5 scaffolds the nucleolus

Everyone has that one friend who's the life of the party, bringing people together and keeping everyone connected. Now, researchers from Japan find that an unusually structured protein plays a similar role in bringing a diverse group of proteins together and keeping them connected and functional.
In a study published recently in Nature Communications, researchers from Tokyo Medical and Dental University (TMDU) have revealed that an intrinsically disordered protein (IDP) is crucial for the stability of an organelle called the nucleolus.
The nucleolus is critical for transcribing ribosomal DNA, which encodes crucial components of the ribosome, an essential organelle for cellular maintenance, differentiation, and stress responses. Many proteins that are part of the nucleolus are IDPs that are susceptible to deformation and dysfunction in response to stressors such as temperature changes, low oxygen conditions, or dehydration.
"We previously identified the IDP polyglutamine binding protein 5 (PQBP5), also known as nucleolar protein 10 (NOL10), in a screen for proteins that bind to polyglutamine (polyQ) tract sequences in proteins that cause polyQ diseases," says lead author of the study Xiaocen Jin. "PQBP5/NOL10 was later found to be a component of the nucleolus. the integrity of which has been implicated in the pathophysiology of neurodegenerative diseases."
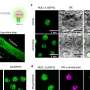
To determine whether PQBP5/NOL10 is involved in maintaining the structural integrity of the nucleolus, the researchers investigated its molecular characteristics, nucleolar sublocalization, relationship with other nucleolar proteins, and stress responses.
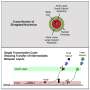
"Unexpectedly, we found that PQBP5/NOL10 is a core structural element of the nucleolus, forming a meshwork that supports other nucleolar substructures," states Hitoshi Okazawa, senior author. "Even more intriguingly, unlike other nucleolar proteins that disperse to the nucleoplasm under osmotic stress conditions, PQBP5/NOL10 remains in the nucleolus and anchors reassembly of the nucleolar structure."
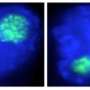
In addition, the researchers found that PQBP5/NOL10 can essentially be sponged up by polyQ disease proteins, both in cells and in mice. This leads to deformation or even disappearance of the nucleoli.
"Taken together, these findings indicate that PQBP5/NOL10 is an essential protein needed to maintain the structure of the nucleolus," says Jin.
Given that polyQ proteins form aggregates with a dense core that often excludes polyQ-binding proteins, PQBP5/NOL10 may initially interact with soluble forms of these proteins before being pulled into larger inclusions. Aggregation inhibitors that prevent inclusion formation could therefore affect PQBP5/NOL10 distribution, and thus nucleolar stability, providing a novel approach to treating polyQ diseases.
More information: Xiaocen Jin et al, PQBP5/NOL10 maintains and anchors the nucleolus under physiological and osmotic stress conditions, Nature Communications (2023).
Journal information: Nature Communications
Provided by Tokyo Medical and Dental University