Researchers reveal endoplasmic reticulum‚Äďassociated protein degradation and control of grain size in rice
In a study published in The Plant Cell, researchers led by Li Yunhai at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences described a role for the endoplasmic reticulum (ER)‚Äďassociated protein degradation in the regulation of grain size in rice (Oryza sativa).
Grain size affects yield and is therefore an important agronomic trait, but our knowledge of the molecular and genetic mechanisms regulating grain size remains limited. Prof. Li's team focused on the molecular networks of grain size control. Recent studies have shown that the ubiquitin-proteasome pathway has an important role in seed size regulation.
Ubiquitination requires the action of a series of special enzymes: ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s). At the same time, the ubiquitin or ubiquitin chain can be removed by deubiquitinating enzymes.
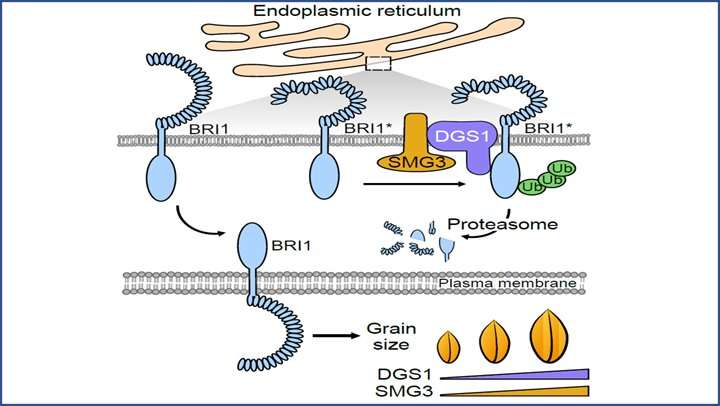
They recently identified an ER‚Äďassociated degradation (ERAD)-related E2-E3 enzyme pair, SMALL GRAIN 3 (SMG3) and DECREASED GRAIN SIZE 1 (DGS1), that regulates grain size and weight through the brassinosteroid (BR) (an important phytohormones) signaling pathway in rice.
ERAD is a special ubiquitin proteasome system that constantly monitors the folding status of secretory and membrane proteins and degrades irreparable terminally misfolded proteins. Specifically, only proteins that form correct and stable conformation can reach the designated localization through the inner membrane system to fulfill biological functions, while defective, misfolded or incompletely folded proteins are retained in the ER and targeted for destruction by ERAD. During this process, the E2-E3 pairs in ER play a key role in the ubiquitination, translocation and degradation of the defective proteins.
SMG3 is a homolog of the Arabidopsis Ubiquitin conjugase UBC32, an E2 ubiquitin-conjugating enzyme involved in the ERAD pathway. DGS1 has been previously characterized in the control of grain size regulation. SMG3 and DGS1 interact with each other in ER and exhibited very similar expression pattern in developing panicles and spikelet. Loss of function of SMG3 or DGS1 results in small grains, whereas overexpression of SMG3 or DGS1 leads to long grains.
Further analysis showed that DGS1 ubiquitinates the BR receptor BRI1 and affects its accumulation, establishing a direct molecular link between ERAD and BR signaling.
"This work provides evidence for the involvement of ER-associated degradation in plant hormone signaling in the control of reproductive development, providing insight into the internal mechanism of seed size control," said Humberto Herrera-Ubaldo, an assistant features editor of The Plant Cell.
More information: Jing Li et al, An endoplasmic reticulum-associated degradation‚Äďrelated E2‚ÄďE3 enzyme pair controls grain size and weight through the brassinosteroid signaling pathway in rice, The Plant Cell (2022).
Journal information: Plant Cell
Provided by Chinese Academy of Sciences