Researchers investigate role of water molecules in formation of condensates in cells
In order to fulfill their function, biological cells need to be divided into separate reaction compartments. This is sometimes done with membranes, and sometimes without them: the spontaneous segregation of certain types of biomolecules leads to the formation of so-called condensates. Why and under which circumstances they form is currently being researched.
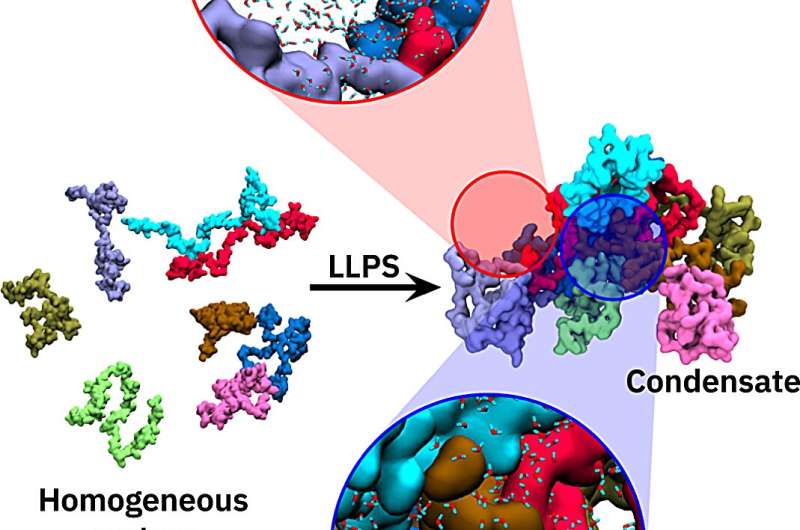
Using computer simulations, Professor Lars Schäfer and Dr. Saumyak Mukherjee from the Center for Theoretical Chemistry at Ruhr University Bochum, Germany, have identified an often overlooked player: water. Because of their sheer numbers, the small water molecules are just as important as the large biomolecules in the molecular tug-of-war of the driving forces that underlie the formation of the condensates. The two researchers describe their findings in an article published in the journal on September 21, 2023.
It wasn't until recently that studies proved the existence of the condensates as reaction spaces in cells. "These condensates are incredibly densely packed, which means that there is a molecular scrum of biological macromolecules such as proteins and nucleic acids," explains Schäfer.
Since only certain macromolecules form such condensates with each other, they can act as specific microreactors for very specific biochemical reactions that take place in the cell. "It's therefore not surprising that disruptions in these processes are associated with various diseases," says Schäfer.
Why do these condensates form in the cell, and under what circumstances? "The underlying driving forces are ultimately hidden in the chemical interactions between the different molecules in the cell," says Mukherjee. "Computer simulations can help shed light on this phenomenon, even in atomic detail." As it turns out, a frequently overlooked player in the molecular interaction has a key role: water.
The properties of the water molecules found in the dense scrum inside the condensates differ from those of the water molecules outside. "The confinement of the water molecules inside the condensate is an unfavorable driving force, while the freedom of the water molecules outside is favorable. The latter win this molecular tug-of-war—if only by a narrow margin," explains Schäfer.
In addition to the frequently described interactions between macromolecules such as proteins, water molecules also play an important role in the formation of biomolecular condensates in cells. "It's a bit like David and Goliath," illustrates Mukherjee. "Here are the small water molecules and there are the big protein molecules. However, there are a lot of water molecules, and together they add as much to the driving force as the large proteins."
More information: Saumyak Mukherjee et al, Thermodynamic forces from protein and water govern condensate formation of an intrinsically disordered protein domain, Nature Communications (2023).
Journal information: Nature Communications
Provided by Ruhr-Universitaet-Bochum