Safely removing nanoplastics from water using 'Prussian blue', a pigment used to dye jeans
Plastic waste breaks down over time into nanoplastics (<0.1 μm). Microplastics smaller than 20 μm cannot be removed in currently operating water treatment plants and must be agglomerated to a larger size and then removed. Iron (Fe) or aluminum (Al) based flocculants are used for this purpose, but they are not the ultimate solution as they remain in the water and cause severe toxicity to humans, requiring a separate treatment process.
Dr. Jae-Woo Choi of the Center for Water Cycle Research at the Korea Institute of Science and Technology (KIST) has developed an eco-friendly metal-organic skeleton-based solid flocculant that can effectively aggregate nanoplastics under visible light irradiation. The research was published in Water Research.
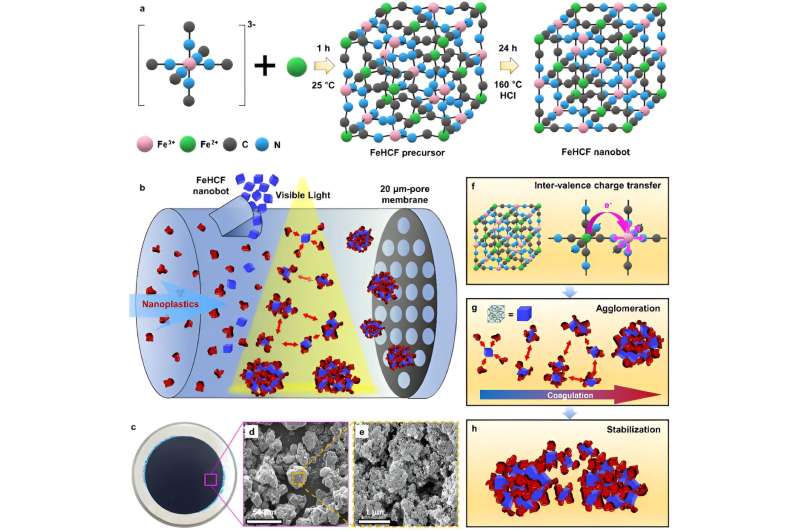
Prussian blue, a metal-organic frameworks-based substance made by adding iron (III) chloride to a potassium ferrocyanide solution, is the first synthetic pigment used to dye jeans a deep blue color and has recently been used to adsorb cesium, a radioactive element, from Japanese nuclear plant wastewater.
While conducting experiments on the removal of radioactive materials from water using Prussian blue, the KIST research team discovered that Prussian blue effectively aggregates nanoplastics under visible light irradiation.
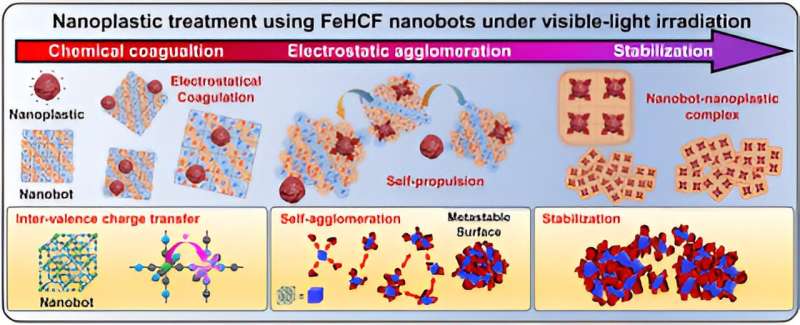
The research team developed a material that can effectively remove nanoplastics by adjusting the crystal structure to maximize the aggregation efficiency of Prussian blue. When the developed material is irradiated with visible light, nanoplastics with a diameter of about 0.15 μm (150 nm), which are difficult to remove using conventional filtration technology, can be agglomerated to a size about 4,100 times larger, making them easier to remove.
In experiments, the researchers found that they were able to remove up to 99% of nanoplastics from water. The developed material is also capable of flocculating nanoplastics more than three times its own weight, outperforming the flocculation efficiency of conventional flocculants using iron or aluminum by about 250 times.
The material not only uses Prussian blue, which is harmless to the human body, but is also a solid flocculant, making it easy to recover residues in water. It also uses natural light as an energy source, enabling a low-energy process.
"This technology has a high potential for commercialization as a candidate material that can be applied to general rivers, wastewater treatment facilities, and water purification plants," said Dr. Choi of KIST. "The developed material can be utilized not only for nanoplastics in water, but also to clean up radioactive cesium, thus providing safe water."
Dr. Youngkyun Jung, the first author of the paper, said, "The principle of this material can be utilized to remove not only nanoplastics, but also a variety of contaminants in water systems."
More information: Youngkyun Jung et al, Visible-light-induced self-propelled nanobots against nanoplastics, Water Research (2023).
Journal information: Water Research
Provided by Korea Institute of Science and Technology