How aromatic dissolved organic matter affects organic micropollutant adsorption
Activated carbon is employed for the adsorption of organic micropollutants (OMPs) from water, typically present in concentrations ranging from ng L−1 to μg L−1. However, the efficacy of OMP removal deteriorates considerably due to competitive adsorption from background dissolved organic matter (DOM), present at substantially higher concentrations in mg L−1. Interpreting the characteristics of competitive DOM is crucial in predicting OMP adsorption efficiencies across diverse natural waters.
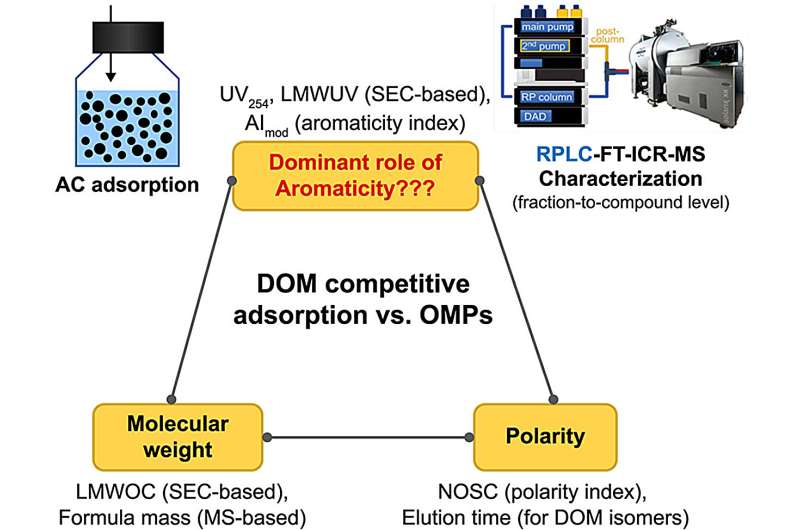
In a study in Environmental Science and Ecotechnology, a multi-national team describes the intricate influence of aromaticity and polarity in low MW DOM competition, from a fraction level to a compound level. They achieved this by employing direct sample injection liquid chromatography coupled with ultrahigh-resolution Fourier-transform ion cyclotron resonance mass spectrometry.
Anion exchange resin pre-treatment eliminated 93% of UV254-active DOM, predominantly aromatic and polar DOM, and only minimally alleviated DOM competition. Molecular characterization revealed that nonpolar molecular formulas (constituting 26% PAC-adsorbable DOM) with medium aromaticity contributed more to the DOM competitiveness.
Isomer-level analysis indicated that the competitiveness of highly aromatic LMW DOM compounds was strongly counterbalanced by the increased polarity. These findings suggest that aromatic DOM (as measured by UV254) was not essentially competitive against OMPs in adsorption.
The study illustrates the counterbalancing effect of aromaticity and polarity in understanding the competitive adsorption of DOM and highlights the limitations of relying solely on aromaticity or UV254-based methods as the sole interpretive metric.
More information: Qi Wang et al, How aromatic dissolved organic matter differs in competitiveness against organic micropollutant adsorption, Environmental Science and Ecotechnology (2024).
Provided by Eurasia Academic Publishing Group