Researchers realize electrochemical conversion of CH4 and O2 to HCOOH at room temperature. Credit: Journal of the American Chemical Society
Direct conversion of CH4 and O2 to value-added chemicals is important for natural gas industries. However, challenges remain due to the difficulty of O2 activation in forming active oxygen species for CH4 activation under mild conditions.
Recently, a research group led by Prof. Deng Dehui, Assoc. Prof. Cui Xiaoju and Yu Liang from the Dalian Institute of Chemical Â鶹ŇůÔşics (DICP) of the Chinese Academy of Sciences (CAS) realized the electrochemical conversion of CH4 by O2 to HCOOH at room temperature. This study was in Journal of the American Chemical Society.
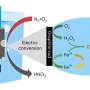
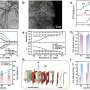
The researchers developed a high-pressure electro-Fenton strategy to establish a hetero-homogeneous process for electro-catalytic conversion of CH4 by O2 at room temperature. They revealed that CH4 was efficiently activated by ·OH, which was produced via a heterogeneous electroreduction of O2 to H2O2 on the Ag foil cathode, followed by a homogeneous Fe2+-facilitated H2O2 decomposition.
Additionally, the researchers found that the elevated pressure not only improved the productivity of H2O2 from O2 electro-reduction but also boosted the reaction collision probability between CH4 and active ·OH in-situ generated from Fe2+-facilitated decomposition of H2O2.
Compared with the traditional electro-catalytic CH4 conversion process with high overpotential (>0.9 V) and low Faradaic efficiency (< 60%), the high-pressure electro-Fenton process achieved an HCOOH Faradaic efficiency of 81.4% with an ultra-low cathodic overpotential of 0.38 V. The HCOOH productivity was 11.5 mmol h-1 gFe-1, which was 220 times that of ambient pressure.
"This work provides a new way for energy-efficient and sustainable conversion of CH4 by directly using O2 under mild conditions," said Prof. Deng.
More information: Yao Song et al, High-Pressure Electro-Fenton Driving CH4 Conversion by O2 at Room Temperature, Journal of the American Chemical Society (2024).
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences