Study decodes dimerization and antidepressant recognition at noradrenaline transporter

The noradrenaline transporter, also known as the norepinephrine transporter (NET), is a member of the monoamine transporters (MATs) family, which also includes serotonin transporter (SERT) and dopamine transporter (DAT). These transporters collectively regulate neurotransmitter concentrations at synapses and maintain neurotransmitter balance in the body.
MATs are primary targets for psychostimulants and antidepressants, and abnormal NET function is closely linked to neuropsychiatric disorders such as attention deficit hyperactivity disorder (ADHD).
The function of MATs is regulated by cholesterol and lipid-mediated oligomerization. Both NET and SERT have been found to be associated with cholesterol-rich regions in brain tissue and transfected cell lines.
Phosphatidylinositol 4,5-bisphosphate (PIP2) promotes NET dimerization and regulates substrate efflux. However, limited structural information has hindered the understanding of precise binding modes of NET with substrates and antidepressants, the mechanisms of selective recognition of antidepressants by three MAT subtypes, and the precise mechanisms by which cholesterol and lipids regulate NET function and its oligomerization process.
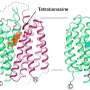
In a study published in , a research team led by Eric H. Xu (Xu Huaqiang) and Yang Dehua from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences, using single-particle cryo-electron microscopy, obtained eight high-resolution structures of the human NET homodimer.
The obtained structures, as the first high-resolution structures of full-length wild-type human NET, included the occluded conformation bound with substrate norepinephrine (NE), the apo state with no substrate bound, and the outward-open conformations bound with six antidepressants (nisoxetine, amitriptyline, maprotiline, nomifensine, tomoxetine, and nefopam), with resolutions ranging from 2.9 to 3.4 angstrom.
Based on these high-resolution structures, researchers revealed that NET forms a homodimer mediated by cholesterol and lipids at the interface, representing a novel mechanism of transporter dimerization. They also revealed how the native substrate norepinephrine binds in the central pocket of NET. In addition, they showed that structures with six different antidepressants illustrate overlapping but distinct binding modes, conferring subtype selectivity over other monoamine transporters.
These findings, by significantly advancing the understanding of NET's structure, regulation, and inhibition, will aid structure-based antidepressant drug design and therapies for neuropsychiatric diseases.
The revelations regarding the unique NET homodimer interface and cholesterol-dependent regulation are of interest to the membrane protein and transporter fields, and provide a clue for the "lipid raft" model of cell membrane.
More information: Heng Zhang et al, Dimerization and antidepressant recognition at noradrenaline transporter, Nature (2024).
Journal information: Nature
Provided by Chinese Academy of Sciences