Study identifies highly soluble molecules with superior antioxidant benefits for cells
Oxygen is essential for life on Earth, but it also gives rise to free radicals, unstable molecules that can damage cells. Antioxidants are chemical compounds that protect cells by neutralizing free radicals. The quintessential antioxidant is ubiquinone, synthesized within cells. However, this molecule is insoluble in water. José VillalaÃn, a professor and researcher at Miguel Hernández University of Elche (UMH), is investigating other molecules with similar antioxidant potential but greater solubility and effectiveness.
from the study, appearing in Free Radical Biology and Medicine, suggest that the molecules under investigation could perform a more comprehensive antioxidant role compared to ubiquinone, which is localized only in certain parts of the membrane.
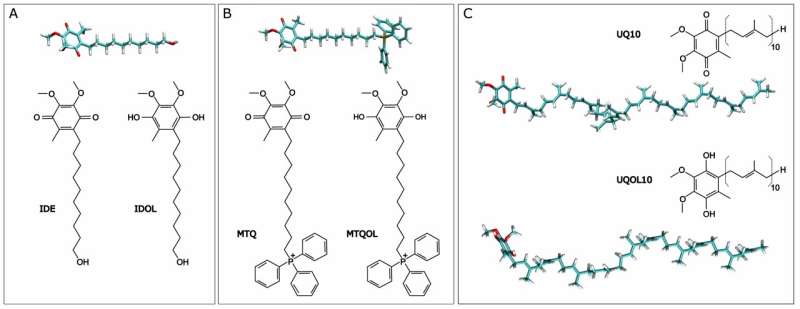
The study focuses on a biomembrane similar to that of mitochondria—a part of all animal cells—and examines the behavior of the molecules idebenone (IDE) and mitoquinone (MTQ).
Ubiquinone is water-insoluble and does not move between membranes without protein transporters. The molecules used in the study are more soluble, can transfer and accumulate, are better absorbed, and can move freely between membranes.
Professor VillalaÃn explains that free radicals affect the body indirectly. The organism cannot function if cells do not work properly, and free radicals increase that risk. However, these harmful compounds are constantly being produced, and cells have mechanisms to control their formation.
Antioxidants help maintain free radicals at a minimum level. Controlling the formation of these damaging compounds can help prevent, in certain cases, some degenerative diseases.
Professor VillalaÃn, who works at the Institute of Research, Development, and Innovation in Health Biotechnology (IDiBE) at UMH, adds that locating the molecules (IDE and MTQ) in different zones and at varying depths of the biological membrane helps reduce free radical production. He emphasizes that the goal is not to replace ubiquinone but to complement it with other antioxidants that function at different membrane levels.
The study was conducted using molecular dynamics, a "virtual simulation" process requiring significant computing power, necessitating a cluster of computers for the experiment.
These simulations determined the location and interaction of the studied molecules, both in their oxidized and reduced forms, in a membrane similar to that of mitochondria. For such research, UMH utilizes a scientific computing cluster, a network of high-speed interconnected computers managed by the Innovation and Technological Planning Service.
More information: José VillalaÃn, Location and interaction of idebenone and mitoquinone in a membrane similar to the inner mitochondrial membrane. Comparison with ubiquinone 10, Free Radical Biology and Medicine (2024).
Journal information: Free Radical Biology and Medicine
Provided by Miguel Hernandez University of Elche