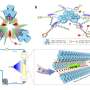
Photocatalytic H2O2 production by using only water and oxygen as ingredients is an attractive and sustainable strategy to replace traditional anthraquinone process. Using a BaCl2-mediated in-plane polymerization strategy, researchers synthesized a novel Ba-implanted graphitic carbon nitride photocatalyst with unique highly ordered nanorod structures, which demonstrated a boosted photocatalytic H2O2-evolution rate. This innovation paves the way for greener and more efficient H2O2 production process. Credit: Chinese Journal of Catalysis
H2O2, a green oxidant and clean fuel, is in high demand across chemical industries, environmental treatments, and even aerospace. However, traditional production methods rely on energy-intensive processes that are not environmentally friendly. Scientists have been seeking a greener alternative, and semiconductor photocatalysis using solar energy to drive chemical reactions has emerged as a promising solution.
To date, various photocatalysts, such as TiO2, BiVO4, metal-organic complexes and organic polymers, have been explored for H2O2 photosynthesis. Cost-effective graphitic carbon nitride (g-C3N4) has caught widespread attention in H2O2 photosynthesis due to its elemental abundance, high structure stability and appropriate band structure.
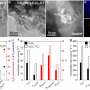
However, the H2O2 production efficiency of traditional g-C3N4 is severely limited due to poor crystallinity and selectivity in two-electron oxygen reduction reaction (2e--ORR). Therefore, improving the in-plane crystallinity of bulk g-C3N4 is greatly requisite to sufficiently trigger oxygen reduction reaction for efficient photocatalytic H2O2 production.
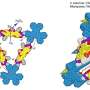
Recently, a research team led by Prof. Yaorong Su from Shenzhen Technology University, China, ingeniously overcomes these obstacles. They developed a new type of photocatalyst, in-plane highly ordered g-C3N4 nanorods with barium (Ba) atoms implanted. This innovation not only enhances the in-plane crystallinity but also induces a highly selective 2e--ORR, which is the key to efficient H2O2 production.
The Ba-implanted nanorods work their magic by altering the way oxygen molecules (O2) interact with the photocatalyst. Instead of the typical side-on binding that favors water production, the Ba atoms encourage a more favorable end-on binding. This change significantly reduces the possibility of O-O bond breaking, effectively suppressing the competing four-electron reaction and boosting the production of H2O2.
An outstanding 6.1 times increase in H2O2 production rate compared to the original g-C3N4 was achieved. This breakthrough not only optimizes the photocatalytic process of solar energy-driven H2O2 photosynthesis but also opens up new possibilities for designing efficient catalysts for solar-to-fuel conversion, bringing us closer to a sustainable future.
This work was published in the .
More information: Aiyun Meng et al, Towards highly-selective H2O2 photosynthesis: In-plane highly ordered carbon nitride nanorods with Ba atoms implantation, Chinese Journal of Catalysis (2024).
Provided by Chinese Academy of Sciences