Metallic nanosheets curl into nanovesicles

Inspired by the cell membrane structure, researchers, led by Dr. Xiaoqing Huang (State Key Laboratory of Â鶹ÒùÔºical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University) and Dr. Qi Shao (College of Chemistry and Chemical Engineering and Materials Science, Soochow University), propose a biomimetic strategy for synthesis of nanovesicles by utilizing the interfacial strain as the driving force to curl the ultrathin nanosheets into nanovesicles.
Meanwhile, the electrocatalyst with the vesicular structure displays excellent HOR activity and stability.
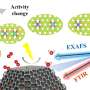
To study the formation mechanism of RhRu nanovesicles, morphologies and compositions of the reaction intermediates were initially collected using transmission electron microscopy (TEM), high-angle annular dark-field scanning TEM (HAADF-STEM) line scan and TEM energy-dispersive X-ray spectroscopy (TEM-EDS) analysis, respectively.
It was found that the pure Rh small nanosheets were formed in the beginning stage; then the small nanosheets grew gradually along the lateral direction.
As the reaction time went on, the Ru element was gradually reduced and the nanosheet began to bend to form a bowl-like structure. Prolonging the reaction time, the bowl-like structure gradually formed a vesicle structure.
The researchers conclude that the introduction of Ru atoms plays a critical role in the curling growth of the nanosheet structure. Meanwhile, density functional theory (DFT) calculations reveal that the Ru atoms make the curling of the nanosheet more favorable in thermodynamics.
Owing to the unique vesicular structure, the RhRu nanovesicles/C displays excellent hydrogen oxidation reaction (HOR) activity and stability.
The mass activity of RhRu nanovesicles/C at an overpotential of 50 mV is 7.50 A mg(Rh+Ru)−1, which is 24.19 times higher than that of Pt/C (0.31 A mgPt−1). Moreover, the RhRu nanovesicles/C-based membrane electrode assembly (MEA) displays a high peak power density (PPD) of 1.62 W cm−2, possessing a potential application in hydroxide exchange membrane fuel cell (HEMFC).
This work shows that it is feasible to design new structures by the biomimetic strategy, which can draw rapid attention in biomimetic synthesis.
The research is in the journal National Science Review.
More information: Juntao Zhang et al, An all-metallic nanovesicle for hydrogen oxidation, National Science Review (2024).
Provided by Science China Press