Discovery of a stable single-electron covalent bond between two carbon atoms validates a century-old theory

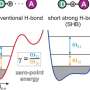
Covalent bonds, in which two atoms are bound together by sharing a pair of electrons, form the scaffolding that underpins the majority of organic compounds. In 1931, the Nobel Laureate Linus Pauling suggested that covalent bonds made from just a single, unpaired electron could exist, but these single-electron bonds would likely be much weaker than a standard covalent bond involving a pair of electrons.
Since then, single-electron bonds have been observed, but never in carbon or hydrogen—the hunt for one-electron bonds shared between carbon atoms has stymied scientists.
Now, a team of researchers from Hokkaido University has isolated a compound in which a single electron is shared between two carbon atoms in a remarkably stable covalent bond, known as a sigma bond. Their findings are in the journal Nature.
"Elucidating the nature of single-electron sigma-bonds between two carbon atoms is essential to gain a deeper understanding of chemical-bonding theories and would provide further insights into chemical reactions," explains Professor Yusuke Ishigaki, of the Department of Chemistry at Hokkaido University, who co-authored the study.

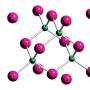
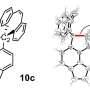
The single-electron bond was formed by subjecting a derivative of hexaphenylethane, which contains an extremely stretched out paired-electron covalent bond between two carbon atoms, to an oxidation reaction in the presence of iodine. The reaction produced dark violet-colored crystals of an iodine salt.
The team used X-ray diffraction analysis to study the crystals and found that the carbon atoms in them were extremely close together, suggesting the presence of single-electron covalent bonds between carbon atoms. They were then able to confirm this using a form of chemical analysis called Raman spectroscopy.
"These results thus constitute the first piece of experimental evidence for a carbon-carbon single-electron covalent bond, which can be expected to pave the way for further developments of the chemistry of this scarcely-explored type of bonding," Takuya Shimajiri, the lead author of the paper and now at the University of Tokyo, says.
More information: Takuya Shimajiri et al, Direct evidence for a carbon–carbon one-electron σ-bond, Nature (2024). .
Journal information: Nature
Provided by Hokkaido University