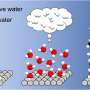
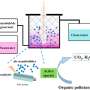
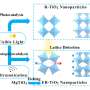
Graphical abstract. Credit: The Journal of Â鶹ÒùÔºical Chemistry C (2024). DOI: 10.1021/acs.jpcc.4c05777
A study led by Dr. Marcelo I. Guzman and his team at the University of Kentucky has unveiled significant advancements in the field of photocatalysis. The research, in the Journal of Â鶹ÒùÔºical Chemistry C, explores the photocatalytic behavior of catechol adsorbed on Degussa P25 TiO2 at the air-solid interface. The work offers new insights into environmental remediation and sustainable chemical processes.
The research focuses on the photocatalytic degradation of catechol, a common organic pollutant, when adsorbed on Degussa P25 TiO2, a widely used photocatalyst. By conducting experiments at the air-solid interface, the team observed a significant increase in photocatalytic efficiency compared to traditional aqueous-phase reactions. This is attributed to the unique interaction dynamics and surface properties at the air-solid interface, which facilitate more effective light absorption and reactive species generation.
The study meticulously examines the interaction between catechol and TiO2, highlighting the enhanced photocatalytic activity under relevant environmental conditions. This innovative approach not only improves our understanding of photocatalytic mechanisms but also opens up new avenues for practical applications in pollution control and green chemistry.
Dr. Guzman commented, "Our findings demonstrate the potential of ligand-to-metal charge transfer complexes of TiO2 as photocatalysts for addressing environmental challenges. This research paves the way for developing more efficient and sustainable technologies for air and water purification."
Diffuse Reflectance UV-visible spectra (DRUVS) of TiO2/catechol film irradiated at λcut-off ≥ 515 nm during (pink) 2 min fitted with four Gaussian functions for peaks centered at (blue, peak 1) 439.0 nm, (purple, peak 2) 458.5 nm, (green, peak 3) 591.0 nm, (cyan, peak 4) at 699.0 nm, and (red) the composite simulated spectrum of the Gaussian fittings. Credit: The Journal of Â鶹ÒùÔºical Chemistry C (2024). DOI: 10.1021/acs.jpcc.4c05777
A key finding of the study includes showing an enhanced photocatalytic degradation of catechol at the air-solid interface. This is due to the increased availability of reactive oxygen species (ROS) generated under light irradiation.
The work demonstrates detailed reaction mechanisms with the participation of free radical intermediates, providing a deeper understanding of the photocatalytic pathways involved. The identification of these intermediates is crucial for optimizing the photocatalytic process and minimizing the formation of potentially harmful by-products. The work is an important scientific contribution with potential applications in environmental cleanup and sustainable industrial processes.
The research team comprises chemists that applied their expertise in environmental, physical, analytical, electrochemical, photocatalysis, and atmospheric sciences, ensuring a comprehensive approach to the study. Their collaborative efforts underscore the importance of interdisciplinary research in tackling complex scientific problems.
More information: Md Ariful Hoque et al, Photocatalysis of Adsorbed Catechol on Degussa P25 TiO2 at the Air–Solid Interface, The Journal of Â鶹ÒùÔºical Chemistry C (2024).
Journal information: Journal of Â鶹ÒùÔºical Chemistry C
Provided by University of Kentucky