Advance in photochemical water oxidation enhances sustainable energy potential

With the global shift towards sustainable and renewable energy, the urgency to develop efficient methods for producing clean energy has never been greater. Imagine a future where the energy that powers our homes and cities comes from one of the planet's most abundant resources—water.
Scientists are turning this vision into reality through photochemical water oxidation, a process that uses light to split water molecules, releasing oxygen and enabling clean, sustainable energy. Water oxidation holds enormous potential, but the dependence of catalytic activity with different catalysts behind this reaction are not yet fully understood.
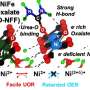
To unlock its full potential, researchers from the Institute of Science Tokyo, led by Assistant Professor Megumi Okazaki, are actively investigating the factors that drive this process. Their study was published in the journal . This research work uncovers the key elements that govern the efficiency of water splitting, focusing on the role of Ru(II) photosensitizers, metal oxide (MOx) catalysts, and pH conditions.
Researchers investigated the performance of Ru(II) photosensitizers paired with various MOx catalysts under different pH conditions. They employed a novel approach to estimate the reaction potential (EMOx) of the catalysts without requiring complex electrochemical setups. Data were analyzed to identify the thresholds at which oxygen evolution began and to evaluate how the potential gap between the photosensitizer and catalyst influenced the efficiency.
The study identified several factors that influence water oxidation efficiency. "Reaction potential (EMOx) plays a critical role in the water oxidation process, directly visualizing the driving force towards water oxidation that have never measured by any apparatus under reaction condition," shares Okazaki.
The results also show that the onset pH conditions, which is whether water oxidation proceeds or not, vary across different MOx catalysts, highlighting the importance of tailoring reaction environments for each catalyst. The study also emphasized the importance of threshold potential— the point at which oxygen production begins for each catalyst, marking the initiation of the reaction.
The study confirmed that fine-tuning reaction potential and pH conditions can significantly enhance the efficiency of water oxidation. By identifying optimal conditions for each catalyst, it provides a strategic framework for designing more effective systems.
Okazaki explains, "By developing a simplified method to estimate reaction potentials, we are making this research more accessible and cost-effective. This innovation could revolutionize the way we design and select catalysts, accelerating progress toward more efficient and sustainable energy solutions."
These findings offer stepping stones toward a more sustainable future. By optimizing the reaction conditions, scientists can create more efficient systems for producing clean energy. This not only reduces reliance on fossil fuels but also makes renewable energy technologies more accessible worldwide. Moreover, the innovative method for estimating reaction potentials could transform how researchers design and select catalysts, speeding up progress in this field.
By exploring the interplay between catalysts, photosensitizers, and pH, this study lays the foundation for more efficient water oxidation systems. It brings us closer to practical solutions for the energy crisis and has the potential to revolutionize clean energy generation. In the pursuit of clean energy, every breakthrough matters—and this one could pave the way to a greener and more sustainable planet.
More information: Megumi Okazaki et al, Discovery of the threshold potential that triggers photochemical water oxidation with Ru(II) photosensitizers and MO catalysts, Chem Catalysis (2024).
Journal information: Chem Catalysis
Provided by Institute of Science Tokyo