Electron microscopy captures enzyme step in antibiotic production

Nonribosomal peptide synthetase (NRPS) enzymes are essential in creating important medications, such as penicillin and cyclosporine. This is done through a multi-step process where the enzymes activate amino acid building blocks and convert them into elongated peptides.
Because of their large size, complex design and changing shapes, NRPS enzymes are highly dynamic and difficult to study. Visualizing specific steps in the process has been challenging because the reactions happen too quickly to capture clear snapshots.
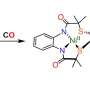
In recent years, the lab of UC San Diego Professor of Chemistry and Biochemistry Michael Burkart has developed crosslinking tools to trap the enzyme at specific steps, freezing them in place. In this work, they focused on peptide condensation of two key modules involved in producing tyrocidine B, a natural antibiotic. The findings are in the journal Nature.
Using electron microscopy, the team was able to visualize the trapped proteins at a specific moment during the reaction and uncovered new ways the proteins coordinate their actions, which helps ensure the pathway runs smoothly.
"Understanding the relationships within these enzymes and how they work can help us in using synthetic biology to design and produce new drug therapies in the future," said postdoctoral scholar Graham Heberlig, who is the paper's lead author.
More information: Graham W. Heberlig et al, Crosslinking intermodular condensation in non-ribosomal peptide biosynthesis, Nature (2024).
Journal information: Nature
Provided by University of California - San Diego