Study reveals new mechanism of mRNA poly(A) tail regulation in early embryos

During the oocyte-to-embryo transition (OET), the length of the mRNA poly(A) tail is closely linked to its translational efficiency. Regulation of the poly(A) tail is essential for selective protein translation in early embryos. However, the mechanisms that determine and maintain the length of maternal mRNA poly(A) tails remain unclear.
A collaborative study in Nature Communications on Jan. 2 by researchers from the Institute of Biophysics and the Institute of Genetics and Developmental Biology at the Chinese Academy of Sciences unveiled a maternal mRNA poly(A) tail protection mechanism in early embryos.
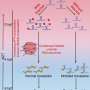
The researchers identified proteins associated with the poly(A) deadenylase complex CCR4-NOT and discovered a poly(A) tail regulatory factor, named MARTRE1, which is selectively expressed in 2-cell-like cells (2CLCs).
Cellular and in vitro biochemical experiments showed that MARTRE1 functions as an inhibitor of the CCR4-NOT complex. When MARTRE1 was expressed in cells, it slowed the rate of poly(A) tail shortening and enhanced mRNA stability.
Homology sequence analysis indicated that Martre1 belongs to a previously under-explored gene family, now designated the Martre gene family.
By constructing a mouse model with a complete knockout of the Martre gene family, the researchers observed delays in early embryonic development in Martre knockout mice.
Combining transcriptomics, translatomics, and third-generation sequencing to measure mRNA poly(A) tail lengths, the researchers demonstrated that MARTRE proteins protect long-tailed mRNAs from excessive deadenylation in early embryos, thereby ensuring efficient translation of maternal mRNAs.
This study identified the first known inhibitor of the CCR4-NOT complex during the OET phase in mammals, offering new insights into the maternal control mechanisms governing early embryonic development.
"By protecting translated mRNAs from deadenylation, the early embryo can sustain efficient translation using limited maternal mRNA, which may be a universal strategy for regulating maternal gene translation during early development across species," said Prof. Bing Zhu.
More information: Jing Yang et al, MARTRE family proteins negatively regulate CCR4-NOT activity to protect poly(A) tail length and promote translation of maternal mRNA, Nature Communications (2025).
Journal information: Nature Communications
Provided by Chinese Academy of Sciences