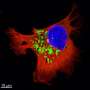
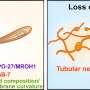
Mechanism of BORC complex assembly in regulating lysosome transport. Credit: Feng Wei's group
A research team led by Prof. Feng Wei at the Institute of Biophysics, Chinese Academy of Sciences, has made significant strides in understanding the BORC complex, a key player in lysosome transport and localization.
The hetero-octameric complex BORC recruits the small G protein ARL8 to mediate the transport and localization of lysosomes. BORC-ARL8 is an essential regulatory factor in the fusion of lysosomes with autophagosomes and in targeting newly synthesized proteins to the lysosome.
However, the molecular mechanism by which the BORC complex assembles and interacts with ARL8 has remained unclear.
Now, Feng and his team revealed, for the first time, the coiled-coil structure of the BORC hetero-octameric complex and identified key regions involved in its binding to ARL8. These findings provide a solid foundation for further exploration of the regulatory mechanisms underlying lysosome transport. Their findings were in Structure on Dec. 30, 2024.
Using cryo-electron microscopy (cryo-EM), the researchers discovered that the BORC complex does not adopt the anticipated "bead-on-a-string" configuration; instead, it exhibits a coiled-coil assembly pattern, a structure that presents significant challenges in structural biology.
To overcome technical challenges such as severe orientation bias in cryo-EM samples and pronounced anisotropy in crystal diffraction, the researchers employed crosslinking mass spectrometry and AlphaFold structural prediction methods. This innovative approach allowed them to successfully resolve the structure of the BORC hetero-octameric complex.
Their results revealed that the eight subunits of BORC form an elongated rod-like structure. The BORC holocomplex is assembled from two hemicomplexes joined end-to-end, with each hemicomplex consisting of four subunits (BORCS1/4/6/8 or BORCS2/3/5/7), which also organize into coiled-coil bundles.
The study further revealed that BORC can sequentially recruit additional subunits through its core sub-complex of four subunits (BORCS1/2/3/5), suggesting the presence of several biologically significant sub-complexes in vivo.
Through crosslinking mass spectrometry, the researchers identified that BORC interacts with ARL8 via a peptide sequence located at the N-terminus of the BORCS5 subunit. To validate this finding, they created a BORCS5 knockout cell line using CRISPR/Cas9 and confirmed, through rescue experiments, that this interaction is critical for lysosome transport.
"Lysosomal dynamic transport is essential for the growth and invasion of cancer cells and represents a potential target for anti-cancer therapies," said Prof. Feng. "Our study provides important theoretical support for the biological study of lysosome transport and localization."
More information: Xuan Ge et al, The structure and assembly of the hetero-octameric BLOC-one-related complex, Structure (2024).
Journal information: Structure
Provided by Chinese Academy of Sciences