Biomolecular 'silly putty': High-res imaging of cellular condensates achieved using fluorogen technology

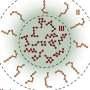
Biomolecular condensates are shifting blobs in our cells that organize cellular matter. They are distinct molecular communities made of DNA, RNA and proteins that "condense" molecules to key locations, yet they frequently defy description. Partly this is because they are so small, they cannot be measured using traditional microscopes.
"These blobs were once described as being 'liquid-like' because some of them were observed to kiss, fuse, drip and flow like raindrops on windshields," said Rohit Pappu, Gene K. Beare Distinguished Professor of biomedical engineering the McKelvey School of Engineering at Washington University in St. Louis.
However, while the blobs may look like raindrops, computations have suggested otherwise. The molecular organization within condensates is more like that of a network that rearranges on different timescales, giving condensates more of a shifting, silly putty-like character.
Working with the lab of Matthew Lew, associate professor of electrical and systems engineering at McKelvey, Pappu and colleagues, all members of the Center for Biomolecular Condensates at WashU, tested computational predictions by peering into condensates using novel super-resolution microscopy methods.
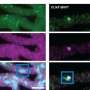
In research published in , the Pappu and Lew labs show how to employ fluorogens—environmentally sensitive dyes that only light up in certain chemical environments—to peer into condensates at high resolutions that scientists have previously been unable to achieve. They used these fluorogens one at a time, aided by unique imaging technologies developed in the Lew lab, to peer into condensates at high resolutions.
These advances in imaging are central to understanding how condensates function and how they go awry in the context of cancers and neurodegenerative diseases, which are associated with functionally aberrant condensates.
Existing techniques rely on averaging the behavior of the whole ensemble of molecules within condensates. In contrast, this new single fluorogen method allows single and multimolecular resolution. The WashU team utilized fluorescent chemical probes that only light up if they encounter the right kind of chemical and viscoelastic environment, serving as beacons for regions that appear as hubs within networks of molecules that are held together by "stickers."
About those stickers: If you think of condensates as a group of people, the stickers are the friends who make the decisions about where and when to gather and whom to invite, the researchers said.
"Enabled by the interactions written into protein sequences, certain individual proteins are the hubs of the viscoelastic (silly putty) network structure within the condensate," Lew said. "Our fluorogen sensors won't light up until they've found these hubs. Tracking the movements of individual fluorogens enabled us to find and track the hubs as they formed, moved and disassembled."
Pappu described the microscopy as being akin to sending a single ant to map and navigate a dark house. The ant will spend more time around sections where sugar has been left out for it and the map it makes will glow brightest around that sugar.
Using a single ant—actually, a single fluorogen—allows researchers to avoid collecting conflicting signals about the terrain that would occur if you watched many ants at the same time—akin to existing techniques.
With that single strong signal and using a super resolution microscope, scientists can now detect single molecules and track their movements with resolution beyond the diffraction limit.
"The fluorogens swim inside a condensate and help us map the internal organization for the first time," Pappu said. "This was made possible by Matt Lew's innovations and the collaborations enabled by our unique center."
More information: Tingting Wu et al, Single-fluorogen imaging reveals distinct environmental and structural features of biomolecular condensates, Nature Â鶹ÒùÔºics (2025).
Journal information: Nature Â鶹ÒùÔºics
Provided by Washington University in St. Louis