Time-resolved photoluminescence unlocks nanoscale insights into surface-modified metal oxide semiconductors

In the quest for next-generation energy, sensing, and pigment technologies, semiconducting metal oxides like titanium dioxide (TiO₂) have emerged as essential materials due to their abundance, stability, and intriguing photophysical properties. But there's a catch: Their surfaces—where most chemical interactions occur—often behave unpredictably, limiting their performance in applications ranging from photocatalysis to solar energy harvesting.
To optimize these surfaces, researchers have turned to coating strategies—applying ultra-thin inorganic layers that tailor surface behavior without compromising the underlying semiconducting properties. However, one big question remains: How do we see what these coatings are doing at the nanoscale—especially in real-time and with high sensitivity?
Our recent study at the University of Delaware in collaboration with the Chemours Company introduces time-resolved photoluminescence (TRPL) as a powerful and noninvasive optical tool to probe these coatings on semiconducting metal oxides.
For the first time, we demonstrate how TRPL can track the influence of inorganic surface modifiers on the photophysical response of TiOâ‚‚ nanoparticles, offering a window into understanding charge transfer dynamics and surface characterization that were previously elusive. Our research is in The Journal of Â鶹ÒùÔºical Chemistry C.
Shedding light on the problem
TiO₂ is well-known for its wide bandgap and strong photoreactivity. But when it absorbs UV light, it generates electron-hole pairs (excitons) that tend to recombine rapidly—before they can be harnessed for useful reactions like water splitting, photocatalysis or pollutant degradation. This same behavior is also responsible for unwanted photodegradation in paints, paper and plastics where TiO2 is used as pigments. These recombination events predominantly occur at surface sites, which act as traps for charge carriers.
Researchers have tried various surface treatments to passivate these traps, including coating TiO₂ with thin layers of metal oxides like Al₂O₃, ZrO₂, or SiO₂. These inorganic coatings can reduce surface recombination, improve chemical selectivity, and even alter the electronic properties of the surface without altering the bulk properties.
However, directly probing how these coatings—especially as a function of their thickness and coverage—impact charge carrier dynamics at ultrafast timescales has remained a significant challenge.
To address this challenge, we turned to TRPL.

What is time-resolved photoluminescence (TRPL)?
TRPL is a laser-based technique that tracks how long it takes for photoluminescence—light emitted by a material after excitation—to decay over time. These decay times offer insight into how quickly photoexcited charge carriers recombine, get trapped or injected into the conduction band of the semiconductor.
In our study, we used pulsed laser excitation to selectively excite chromophores (light-sensitive dye molecules) bound to TiO₂ nanoparticles, then monitored their emission decay using time-correlated single photon counting (TCSPC) techniques. By comparing the decay profiles of chromophores bound to bare TiO₂ with those of surface-coated variants, we could directly observe how surface modifications—shell thickness and patch coverage—affect charge transfer and recombination behavior on the nanosecond timescale.
Key findings
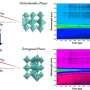
We examined dye-sensitized coated TiO₂ samples with thin, thick and patchy layers of Al₂O₃ using an improved wet chemical deposition method—a technique that enables fine control over coating thickness and morphology. Time-resolved photoluminescence (TRPL) measurements revealed several striking distinctions among the samples.
- Slower decay times: All coated samples exhibited longer photoluminescence lifetimes compared to uncoated TiO₂, indicating reduced surface recombination. This suggests that the Al₂O₃ coatings effectively passivate surface trap states.
- Biexponential decay: The patchy-coated samples showed a two-component decay, suggesting the presence of both fast and slow recombination pathways. This dual behavior was leveraged as a diagnostic to evaluate surface coverage uniformity or quality.
- Monoexponential decay: In contrast, uniformly coated samples exhibited monoexponential decay dominated by slower recombination processes. The decay times increased from 1.8 ns to 3.5 ns as shell thickness increased, highlighting improved carrier lifetimes and enhanced charge separation—beneficial properties for applications like photovoltaics. This relationship served as an optical marker for assessing shell thickness.

Together, these insights demonstrate the power of TRPL in characterizing not just the presence but the quality and extent of surface modifications in semiconducting oxides, offering a valuable tool for rational interface engineering.
Why this matters
Understanding and controlling surface interactions in metal oxides is critical for improving devices that rely on charge transfer at interfaces. This includes:
- Photocatalysts, where surface recombination often limits quantum efficiency.
- Dye-sensitized solar cells, where electron injection and recombination occur at the oxide interface.
- Photoelectrochemical sensors, where surface reactions define selectivity and sensitivity.
By using TRPL to "watch" what happens at these critical interfaces, we can rationally design better coatings, select appropriate materials, and even monitor degradation or aging effects over time.

Broader impacts
Beyond TiO₂, this methodology can be extended to a wide range of wide-bandgap oxides such as ZnO, SnO₂, and WO₃. It's particularly valuable in scenarios where traditional characterization techniques like XPS or TEM fall short—either because they lack time resolution, limited scalability or inability to sensitively capture subtle electronic changes at the surface.
Furthermore, TRPL is nondestructive and can be applied in ambient or controlled environments, making it suitable for in-situ and operando studies—a growing need in fields like catalysis and flexible electronics.
This study redefines photoluminescence as more than a diagnostic tool—it becomes both a window into charge carrier dynamics and a compass for designing functional surfaces. Through time-resolved photoluminescence (TRPL), we move beyond observation to truly understanding and optimizing semiconducting metal oxides. As surface-driven technologies continue to evolve, one thing is certain: Sometimes, the clearest insights begin with the right pulse of light.
This story is part of , where researchers can report findings from their published research articles. for information about Science X Dialog and how to participate.
More information: Michael Uzu et al, Time-Resolved Photoluminescence for Surface Characterization of Modified Metal Oxides, The Journal of Â鶹ÒùÔºical Chemistry C (2025).
Journal information: Journal of Â鶹ÒùÔºical Chemistry C
Michael Uzu is a Ph.D. candidate in Chemistry at the University of Delaware, specializing in surface science and photophysical characterization of nanomaterials. This article is based on his recent research on time-resolved photoluminescence of modified TiOâ‚‚ surfaces.