Cell movement without myosin: New mobility mechanism challenges dogma

Â鶹ÒùÔºicists from the Universities of Bayreuth and Grenoble have discovered a new mechanism of cell mobility. Their findings challenge the classical dogma that the molecular motor myosin is essential for the movement of mammalian cells.
This insight paves the way for new strategies to control cell movement, with potential implications for the treatment of diseases. The findings are in Â鶹ÒùÔºical Review Letters.
The movement of cells in the human body—known as cell migration—is crucial for immune cells. These cells must move quickly and flexibly in different environments in order to fulfill their role as part of our immune system. For example, white blood cells circulate through the body via the bloodstream. When needed, they exit the blood vessels and migrate independently through tissue to sites where they are required, for instance to combat viruses.
However, cell migration can also be harmful: cancer cells may migrate through the body, invade other tissues, and form metastases. Understanding cell migration is therefore essential for the development of new drugs and treatment strategies.
Among the various cell types found in the bodies of humans and other mammals, immune cells are characterized by their rapid movement. Key components of these cells are thread-like protein structures known as actin filaments, which form a network along the inner side of the cell membrane—the cell's surface. This network is dynamic and constantly being remodeled, as actin building blocks are either added to or removed from the ends of filaments.
According to the classical model, the generation of cell movement also requires the molecular motor myosin. These motors shift filaments against each other and thereby contract the network at the rear of the cell, creating a flow of actin filaments from front to back. This flow enables the cell to move forwards. However, experiments in which myosin activity was biochemically suppressed have shown that cells are also capable of moving independently of this molecular motor.
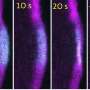
Now, researchers at the Universities of Bayreuth and Grenoble have discovered a mechanism by which cells can achieve this myosin-independent movement. To do so, they developed a model demonstrating that the addition and removal of actin building blocks at the filaments is sufficient to generate cell movement—provided that this process occurs rapidly enough.
Above this critical speed threshold, the distribution of actin filaments within the originally spherical cell changes, leading to the formation of a front and rear that differ in tension: at the front of the cell, where filaments lie less densely beneath the membrane, greater tensions arise than at the rear, where more filaments are present over the same surface area.
This difference causes further actin filaments to flow from the front to the rear, setting up a self-sustaining cycle. In the end, a flow of actin—and thus cell movement—arises even in the absence of molecular motors.
More information: Winfried Schmidt et al, Myosin-Independent Amoeboid Cell Motility, Â鶹ÒùÔºical Review Letters (2025). . On arXiv:
Journal information: Â鶹ÒùÔºical Review Letters , arXiv
Provided by Bayreuth University