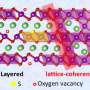
a) SEM image, b) XRD pattern and c) EDS images of LLO material. Credit: Advanced Materials (2025). DOI: 10.1002/adma.202420453
Recently, a research team achieved real-time tracking of electronic/magnetic structure evolution in Li-rich Mn-based materials during the initial cycling through the self-developed operando magnetism characterization device.
Their study, in Advanced Materials, elucidated the critical mechanism underlying the oxygen redox reaction. The research team was led by Prof. Zhao Bangchuan from the Institute of Solid State Â鶹ÒùÔºics, the Hefei Institutes of Â鶹ÒùÔºical Science of the Chinese Academy of Sciences, in collaboration with Prof. Zhong Guohua from the Shenzhen Institute of Advanced Technology and Prof. Li Qiang from Qingdao University.
With the rise of electric vehicles and the low-altitude economy, the demand for high-energy-density batteries is growing. Li-rich Mn-based materials stand out due to their high capacity, wide voltage range, and cost-effectiveness.
However, issues like oxygen release, transition metal migration, and irreversible structural evolution cause voltage decay and capacity loss, limiting practical application. Real-time monitoring of these variations is essential to understanding the oxygen redox mechanism.
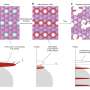
In this study, researchers developed a high-precision operando magnetism testing platform by integrating electrochemical testing with a Superconducting Quantum Interference Device magnetic measurement system, allowing real-time tracking of key structural and electronic transformations in Li-rich Mn-based materials.
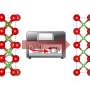
The structure model of Li2MnO3 at different charged states and corresponding projected density of states of Mn and O. Credit: Qiu Shiyu
By observing magnetic changes during charge and discharge cycles, the researchers uncovered dynamic relationships between magnetization, electronic structure, and oxygen interactions—offering new insights into how oxygen redox reaction contributes to specific capacity.
Their findings reveal a two-stage process in magnetization evolution. In the early charging stage below 4.5 V, magnetization decreases as Ni2+ oxidizes to Ni3+/Ni4+, signaling the activation of transition metal redox. When the voltage exceeds 4.5 V, the oxygen redox reaction dominates the charge compensation, leading to an unexpected rebound in magnetization.
This study offers new perspectives on the rational design of high-performance anion redox reaction-based cathode materials, according to the team.
More information: Shiyu Qiu et al, Operando Magnetism on Oxygen Redox Process in Li-Rich Cathodes, Advanced Materials (2025). .
Journal information: Advanced Materials
Provided by Chinese Academy of Sciences