An artificial protein that moves like something found in nature

Sadie Harley
scientific editor

Robert Egan
associate editor

Proteins catalyze life by changing shape when they interact with other molecules. The result is a muscle twitching, the perception of light, or a bit of energy extracted from food. But this crucial ability has eluded the growing field of AI-augmented protein engineering.
Now, researchers at UCSF have shown it is possible to make new proteins that move and change shape like those in nature. This ability will help scientists engineer proteins in powerful new ways to treat disease, clean up pollution, and increase crop yields.
"This study is the first step on a path that will lead far beyond biomedicine, into agriculture and the environment," said Tanja Kortemme, Ph.D., professor of bioengineering and senior author of the study, which appears in .
Scientists have been engineering rigid proteins—proteins that can't move or change shape—since the 1980s. These proteins were first used in commercial products like cleaning solutions.
More recently, they've been employed to produce blockbuster medicines like artificial insulin, GLP-1 weight-loss drugs, and antibody treatments for cancer and inflammation.
While important, these immovable molecules can't match the potential of proteins that can swivel, twist, and morph in complicated ways and then return to their original shape, said Kortemme, who is also an investigator at the Chan-Zuckerberg BioHub San Francisco.
She said the most important proteins to emulate for medical uses are those that regulate processes like metabolism, cell division, and other basic life functions. These powerful proteins are the targets of nearly one in three FDA-approved drugs. They facilitate communication within or between cells by changing from one shape into another, and then back again, like an on-off switch.
An overwhelming problem
Designing such stable yet dynamic forms requires computational power and artificial intelligence that didn't exist until a few years ago.
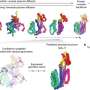
The challenge was huge, so Kortemme and graduate student Amy Guo began with something small: giving a simple natural protein the ability to move in a new way. Guo then made part of the protein swing so it could bind to calcium, a common way that proteins change shape.
"We wanted to devise a design method that could be applied in lots of situations, so we focused on creating a movable part that does what many natural proteins do," she said. "The hope is that this movement could also be added to static artificial proteins to expand what they can do, too."
Guo's next step was to generate a virtual library of thousands of possible shapes that the protein could take. She picked two stable shapes for the protein: one that could bind calcium and another that couldn't.
Then, she zoomed in on specific areas of the virtual protein to look at how the atoms in it were interacting. The work, which began before the pandemic, accelerated once the artificial intelligence program AlphaFold2 became available. Guo used it to make the movable part twist and capture the calcium, and then untwist to set it free.
The moment of truth came when the researchers tested their model in a computer simulation. They teamed up with Mark Kelly, Ph.D., a pharmaceutical chemist at UCSF who uses nuclear magnetic resonance to visualize the atoms in a protein.
"I was amazed that the simulations showed it working exactly like we'd expected it to," Guo said. "That really gives me confidence that this was for real, that we really did it."
In the medical realm, movable engineered proteins could be used in biosensors that change shape in response to signals of disease, triggering an alert. Or they could be used as medicinal proteins that are tailored to work with a person's unique body chemistry.
Shapeshifting proteins could also be designed to break down plastics or help plants resist climate-related stresses like drought or pests. They could even be used to make metal that can repair itself when it cracks.
"The possibilities are truly endless," Guo said.
More information: Science (2025).
Journal information: Science
Provided by University of California, San Francisco