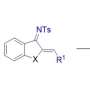
A schematic to show the synthesis of strained, air-stable boracycles via boron–carbon-centered diradicals. Credit: HKUST
Boracycles are important functional scaffolds, finding increasing applications in catalysis, synthesis, materials science, and pharmaceuticals. However, current studies predominantly focus on three-, five-, and six-membered boracycles, leaving four-membered boracycles largely unexplored.
A research team led by Prof. Quan Yangjian and Prof. Lin Zhenyang from the Department of Chemistry at the Hong Kong University of Science and Technology (HKUST), in collaboration with Prof. Lyu Hairong from The Chinese University of Hong Kong (CUHK), has made a breakthrough in developing an efficient synthetic approach to four-membered boracycles. This advancement enables the facile synthesis of other previously inaccessible boracycles, which may lead to valuable applications.
The paper is in the journal Nature Chemistry.
As important structural elements, boracycles demonstrate unique application value in the fields of medicinal chemistry and functional materials. Among them, five-membered and six-membered boron heterocycles have been widely applied in bioactive molecules and optoelectronic materials.
The lack of efficient and versatile synthetic methods has limited the investigation into the properties and applications of four-membered boracycles. Due to their inherent ring strain, four-membered boracycles are expected to serve as valuable synthons with versatile reactivities and functionalities.
Preliminary investigation on the luminescence properties of boracycles. Credit: HKUST
In this recent research, the team developed a novel and efficient synthetic approach to four-membered boracycles, made possible by unlocking boron-carbon diradical (BCDR) chemistry. The triplet energy transfer catalysis creates air-stable benzoboretenes through the intramolecular coupling of BCDR.
Notably, a balance between the stability and reactivity of four-membered boracycles has been achieved for the first time, enabling the facile synthesis of previously inaccessible boracycles. These new boracycles are expected to have valuable applications in boron pharmaceuticals and molecular functional materials.
"More importantly, the concept and strategy of balancing the reactivity and stability of strained boracycles will not only facilitate the development of boracycle synthons but also broaden curiosity-driven research into application-oriented investigations, attracting researchers from diverse fields," Prof. Quan added.
More information: Xinmou Wang et al, Synthesis of strained, air-stable boracycles via boron–carbon-centred diradicals, Nature Chemistry (2025).
Journal information: Nature Chemistry
Provided by Hong Kong University of Science and Technology