Iron carbide: A novel heterogeneous catalyst for deoxygenative C-C coupling of alcohols

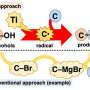
C coupling reactions have received great research interest. In particular, the deoxygenative homocoupling of benzyl alcohols is regarded as one of the most promising routes to produce bibenzyls. Various homogeneous catalytic systems based on transition metal complexes (e.g., Ni, Re, Mo) have been developed for this transformation.
Unfortunately, expensive reductants (e.g., Mn, PPh3) and tedious post-treatment procedures are unavoidable in most cases. These limitations reinforce the need for a heterogeneous catalyst working under hydrogen atmosphere for the direct deoxygenative C-C coupling of benzyl alcohols, producing H2O as the side product.
Inspiration is acquired from the Fischer-Tropsch synthesis (FTS) process, where iron carbides (e.g., Fe5C2) dissociate CO into C* and O* species, and the former then undergo hydrogenation and C-C coupling to yield long-chain hydrocarbons. Therefore, iron carbides may be hopeful catalysts in the deoxygenative coupling process of benzyl alcohols.
Recently, a research team led by Profs. Zhang Tao and Wang Aiqin from the Dalian Institute of Chemical Â鶹ÒùÔºics (DICP) of the Chinese Academy of Sciences (CAS) designed heterogeneous iron carbide catalysts for deoxygenative coupling of benzyl alcohols towards the bibenzyls under a hydrogen atmosphere. The result was in the Chinese Journal of Catalysis.
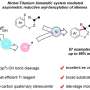
Iron carbide catalysts were prepared through a gas-phase reduction-carbonization route (FeOOH—α-Fe2O3—α-Fe—iron carbide). Different metal dopants in iron carbides were also tested, and Ir0.1/Fe0@Fe5C2 was identified as the optimal catalyst.
This system could be extended to different benzyl alcohols towards various bibenzyls and the introduction of styrene derivatives could switch the reaction mode from homocoupling to the double addition reaction between alcohols and alkenes, yielding a series of polycyclic arenes.
For Ir0.1/Fe0@Fe5C2 catalyst, a core-shell structure with a metallic iron core and Fe5C2 shell was formed, and iridium species existed as nanoparticles. The radical trapping experiment confirmed the radical reaction pathway in this system and the benzyl radical was the key reaction intermediate.
FTIR results collected after benzyl alcohol adsorption verified the promoted C-O bond activation after the carbonization treatment of α-Fe. Ir species accelerated the removal of oxygen species on the catalyst surface during the reaction course and may provide extra C-C coupling sites.
More information: Yichao Wang et al, Iron carbide-catalyzed deoxygenative coupling of benzyl alcohols toward bibenzyls under hydrogen atmosphere, Chinese Journal of Catalysis (2025).
Provided by Chinese Academy of Sciences