Mathematical models and imaging reveal how migrating cells navigate tissue geometry

Sadie Harley
scientific editor

Robert Egan
associate editor

Imagine cells navigating through a complex maze, guided by chemical signals and the physical landscape of their environment. A team of researchers at the University of Maryland, Baltimore County (UMBC) has contributed to an important discovery about how cells move, or migrate, through this maze of bodily tissues, using the fruit fly egg chamber as a model system. Potential implications include better understanding of diseases like cancer and advancing medical treatments.
in iScience, the team's study combines biological experiments and mathematics to reveal new insights into cell migration.
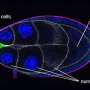
By integrating mathematical modeling with advanced imaging, the team discovered that the physical shape of the egg chamber, combined with chemical signals called chemoattractants, significantly influences how cells move.
"This paper takes an interdisciplinary focus with tight collaboration between a mathematical framework and experimental design," says UMBC mathematician and co-author Brad Peercy.
"The results promote the idea that complex distribution of chemical attractants can explain specific variations in migratory movement."
Peercy's enthusiasm highlights the study's innovative approach, which merges precise mathematical models with real-world biological experiments to uncover patterns that were previously invisible.
Following the breadcrumbs
The team's work focuses on border cells, a type of cell in fruit fly egg chambers, which are a model system for studying cell migration because of their similarities to processes in human development and disease.
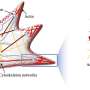
The team found that the border cells' movement wasn't only driven by continuously increasing chemical concentrations from one end of the egg chamber to the other, as earlier models suggested. Instead, the physical structure of the tissue—narrow tubes alternating with wider gaps—played a critical role.
"This was the first time that we characterized that there were these patterns of migration behavior that ended up correlating to aspects of the tissue geometry," explains biologist Alex George, a co-author who completed his Ph.D. at UMBC in 2024 and will begin a postdoctoral fellowship at the Geisel School of Medicine at Dartmouth.
He likens the migration process to Hansel and Gretel following breadcrumbs through a forest: On a flat plain, the trail is clear, but in a landscape with ravines and valleys, the breadcrumbs pool in unexpected ways, complicating the path.
To understand this, co-author Naghmeh Akhavan, who completed her Ph.D. in mathematics at UMBC this spring, developed mathematical models that simulate how cells respond to both chemical signals and tissue geometry together.
"Alex's experiments showed that the speed is not exactly the way previous models showed it," she says. Her models revealed that cells speed up in narrow tubes and slow down in larger gaps, a pattern confirmed by George's imaging.
Both approaches—wet-lab experiments and modeling—bring unique strengths to the work. Putting them together "is like unveiling the invisible from two different perspectives," George says. "My experiments would refine her model, and her model would refine my experiments."
And then, "When our model shows exactly what Alex found in his experiments, we love that," Akhavan adds.
New strategies, new discoveries
The study's broader impact lies in its potential to inform fields beyond developmental biology. Cell migration is critical in processes like wound healing, immune responses, and cancer metastasis.
"Most research on how cells navigate the world has focused only on chemical signals or only on structural ones, so this is one of the first studies to consider how those two things impact each other, which is likely to be relevant in many cases," explains UMBC biologist and co-author Michelle Starz-Gaiano.
By showing how tissue geometry and chemical signals interact, the research could guide new strategies for controlling cell movement via medical treatments.
The team's work continues to evolve, including recent experiments at the Advanced Imaging Center at the Janelia Research Campus in Virginia, where George used specialized microscopes to capture previously unseen dynamics of the relevant chemoattractants. These findings will further refine the team's models, opening new avenues for research.
"We are developing new experimental strategies both on the biology and the math side of things," Starz-Gaiano says, "so it will be exciting to see where this will take us next."
More information: Alexander George et al, Chemotaxis of Drosophila border cells is modulated by tissue geometry through dispersion of chemoattractants, iScience (2025).
Journal information: iScience
Provided by University of Maryland Baltimore County