Molecular modeling predicts fleeting phase where liquids rapidly switch between metal and nonmetal

Sadie Harley
scientific editor

Robert Egan
associate editor

A research team led by Prof. Pavel Jungwirth at the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences (IOCB Prague) has uncovered a previously unknown phenomenon that emerges during the transformation of a liquid from a nonmetal to a conductive metal.
In this transition, they observed a distinct phase in which the system spontaneously and rapidly flips between metallic and nonmetallic states—without settling in either for any meaningful length of time.
This newly proposed theory is grounded in high-level molecular modeling. The study, carried out in collaboration with the University of Oxford, the Faculty of Mathematics and �鶹��Ժics at Charles University, and the J. Heyrovský Institute of �鶹��Ժical Chemistry of the CAS, has been in Nature Communications.
Prof. Jungwirth and his colleagues have long been exploring the conditions under which substances shift from nonmetallic to metallic states. Their research challenges conventional ideas rooted in the fact that metals are typically solid, showing instead that certain liquids can exhibit interesting metallic behavior not present in solids.
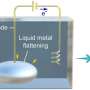
One of their earlier models—where alkali metals such as lithium, sodium, or potassium are dissolved in liquid ammonia until the solution transitions from blue to a golden metallic state—garnered attention in Science five years ago.
As alkali metals release electrons into the solution, the increasing number of free electrons begins to form a conduction band, turning the electrolyte liquid into a liquid metal.
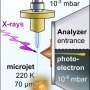
The team at IOCB Prague developed a methodology that not only allows this transition to be calculated but also, in principle, verified experimentally using photoelectron spectroscopy at a synchrotron facility.
Building on these results, they've now used advanced molecular dynamics simulations to propose a new hypothesis: that between the nonmetal and metal phases, there exists a third phase characterized by ultrafast switching—within just tens of femtoseconds—between the two.
"No one had previously realized that a system might oscillate between metallic and nonmetallic states on such a brief timescale. It simply hadn't been considered before," says Prof. Jungwirth. "We believe our theoretical predictions are accurate enough to warrant serious attention."
The team is now working to validate their findings experimentally and is searching for a laboratory equipped to detect these fleeting transitions.
"This won't be easy, because the switching happens incredibly fast—on the order of millionths of a millionth of a second," says Ph.D. student Marco Vitek, the study's first author. "The question is how to capture something that fast in an experiment. Our plan is to use ultrafast lasers, some of which we already have access to here at the institute."
If confirmed, the findings could open a new chapter at the boundary of physics and chemistry—introducing a fundamental physical process not yet covered in the literature.
More information: Marco Vitek et al, Rapid flipping between electrolyte and metallic states in ammonia solutions of alkali metals, Nature Communications (2025).
Journal information: Nature Communications , Science