CRISPR-Cas13 allows selective modification of desired RNA in living cells

Sadie Harley
scientific editor

Robert Egan
associate editor

RNA gene scissors (CRISPR-Cas13) are gaining significant attention as a next-generation gene therapy with fewer side effects. They can suppress infection by eliminating viral RNA, such as in coronaviruses, or regulate the expression of disease-causing genes.
KAIST researchers have developed the world's first technology that can precisely locate and acetylate (chemically modify) only the desired RNA among countless RNA molecules (molecules crucial for transmitting genetic information and producing proteins) within cells. This is expected to be a key technology that could open a new chapter in RNA-based therapies.
This research was in Nature Chemical Biology.
Professor Won Do Heo's research team in the Department of Biological Sciences has developed an innovative technology capable of acetylating specific RNA in the human body using the CRISPR-Cas13 system, an RNA gene scissors system gaining attention in the field of gene regulation and RNA-based technology.
RNA can undergo changes in its properties and functions through a process called "chemical modification"—a gene regulation process where specific chemical groups are added, altering the properties and roles of RNA without changing its nucleotide sequence.
One such chemical modification is cytidine acetylation (N4-acetylcytidine). Until now, the precise function of this chemical modification within cells has not been clearly understood. In particular, there has been ongoing debate about whether this modification truly exists in human mRNA (RNA that produces proteins) and what role it plays.
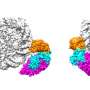
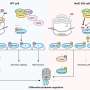
To overcome these limitations, the research team developed a "targeted RNA acetylation system (dCas13-eNAT10)" by combining Cas13, gene scissors that precisely target desired RNA, with a hyperactive variant of NAT10 (eNAT10), an enzyme that acetylates RNA. In essence, they created a "targeted RNA modification technology" that precisely selects and acetylates only the desired RNA.

The research team demonstrated that the targeted RNA acetylation system, guided by guide RNA that locates specific RNA within cells, can introduce acetylation chemical modifications to desired RNA. Through this, they confirm that protein production increases in messenger RNA (mRNA) that has undergone acetylation chemical modification.
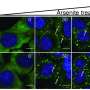
Furthermore, the research team, using the developed system, revealed for the first time that RNA acetylation facilitates the translocation of RNA from the cell nucleus to the cytoplasm. This study demonstrates the possibility that acetylation chemical modification can also regulate intracellular RNA "localization."
The research team also proved that the developed technology could precisely control RNA acetylation within an animal's body by delivering the targeted RNA acetylation system to the liver of experimental mice via AAV (adeno-associated virus), a widely used viral vector in gene therapy.
This is the first case to show that RNA chemical modification technology can be extended to in vivo applications. This achievement is evaluated as opening up possibilities for application in RNA-based gene therapy technology.

Professor Won Do Heo, who previously developed COVID-19 treatment technology using RNA gene scissors and technology to activate RNA gene scissors with light, stated, "Existing RNA chemical modification research faced difficulties in controlling specificity, temporality, and spatiality. However, this new technology allows selective acetylation of desired RNA, opening the door for accurate and detailed research into the functions of RNA acetylation."
"The RNA chemical modification technology developed in this study can be widely used as an RNA-based therapeutic agent and a tool for regulating RNA functions in living organisms in the future."
More information: Jihwan Yu et al, Programmable RNA acetylation with CRISPR–Cas13, Nature Chemical Biology (2025).
Journal information: Nature Chemical Biology