Lipid nanoparticles that engineer CAR T cells in vivo could unlock access for millions of autoimmune patients
Justin Jackson
сontributing writer

Sadie Harley
scientific editor

Robert Egan
associate editor

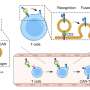
Capstan Therapeutics scientists demonstrate that lipid nanoparticles can engineer CAR T cells within the body without laboratory cell manufacturing and ex vivo expansion. The method using targeted lipid nanoparticles (tLNPs) is designed to deliver messenger RNA specifically to CD8+ T cells.
CAR T therapy has revolutionized the treatment of B-cell cancers, with lasting remissions in lupus, myositis, and leukemia and other B-cell–driven autoimmune disorders. The method requires the use of a patient's own T cells, reengineering those T cells in a lab, growing the population of the modified cells and placing them back in the patient.
More than 20 million U.S. patients living with autoimmune conditions remain without access to the treatment as it depends on costly personalized lab processes, available at only a few specialized centers.
Capstan's lipid-nanoparticle drug is formulated once, then administered to many patients without tailoring the genetic payload for each recipient, making it a universal option without the specialty center price tag or access obstacles.
In the study, "In vivo CAR T cell generation to treat cancer and autoimmune disease," in Science, researchers designed a lipid-nanoparticle delivery system to convert CD8+ T cells inside the body into transient anti-B-cell CAR T cells.
Experiments spanned humanized mice, primary human immune cells, and 22 cynomolgus monkeys given three doses of the therapeutic nanoparticle L829 (0.1–2.0 mg/kg).
A second cohort of 15 monkeys tested a two-dose schedule, and four additional animals examined steroid–antihistamine premedication. Pharmacokinetics, flow cytometry, imaging, and histology tracked CAR expression, B-cell counts, cytokines, and organ distribution.
Bioluminescent and fluorescent reporters confirmed reduced off-target expression in the liver and enhanced accumulation in spleen and lymphoid tissues compared with benchmark lipids used in mRNA vaccines. CD8-L829-tLNPs preferentially modified CD8+ T cells over CD4+ T cells, monocytes, and B cells.
CAR expression was detectable within six hours, declining by 72 hours. CAR T cells engineered in vivo exhibited antigen-specific cytotoxicity, cytokine production, proliferation, and serial killing capacity. Transfected T cells demonstrated effective clearance of CD19+ target cells in vitro.
In humanized mice, a single intravenous dose of 10 or 30 µg CD8-L829-tLNP-CD19 induced near-complete B cell depletion within three hours, with measurable CAR expression on CD8+ T cells persisting at 24 hours.
Nalm6 leukemia-bearing mice receiving the 30 µg dose exhibited near-total tumor clearance in four of five animals within two days of the first dose and complete clearance by the third day after treatment with a second dose.
In cynomolgus monkeys, two or three infusions of CD8-L829-tLNPs encoding an anti-CD20 CAR at 0.1 to 2.0 mg/kg resulted in rapid and profound B cell depletion across blood, spleen, and lymph nodes. CAR expression was observed in up to 85% of CD8+ T cells and 95% of CD8+ NK cells, with minimal expression in CD4+ populations. B cell repopulation began by day 21 and was characterized by a predominantly naive phenotype.
Mild and transient elevations in liver enzymes and pro-inflammatory cytokines, including interleukin-6 and interferon-gamma, were recorded at higher doses. One animal in the 1.5 mg/kg group was euthanized due to clinical features consistent with immune effector cell-associated hemophagocytic lymphohistiocytosis. A compact two-dose regimen replicated depletion efficacy while reducing cytokine release.
Targeted lipid nanoparticles enable in vivo generation of functional CAR T cells without requiring ex vivo cell manipulation or integrating viral vectors.
Researchers at Capstan Therapeutics demonstrated that lipid nanoparticle formulations bearing mRNA and conjugated to CD8-targeting antibodies can program T cells directly inside the body, offering a platform that bypasses conventional manufacturing, lymphodepletion, and the costly infrastructure required for existing CAR T therapies.
As lipid nanoparticle therapeutics are commercially scalable and already validated in mRNA vaccines, this technology may represent a foundational shift toward broader clinical use of engineered immunotherapy from within the patient's body.
Written for you by our author , edited by , and fact-checked and reviewed by —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Theresa L. Hunter et al, In vivo CAR T cell generation to treat cancer and autoimmune disease, Science (2025). .
Journal information: Science
© 2025 Science X Network