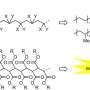
Polymers gain fire resistance and sustainability with light-powered chemical upgrade

Gaby Clark
scientific editor

Robert Egan
associate editor

As demand for advanced polymeric materials increases, post-functionalization has emerged as an effective strategy for designing functional polymers. This approach involves modifying existing polymer chains by introducing new chemical groups after their synthesis, allowing for the transformation of readily available polymers into materials with desirable properties.
Postfunctionalization can be performed under mild conditions using visible light in the presence of catalysts, which provides a sustainable route for developing high-value polymers. However, existing methods often rely on generating carbon radicals along the polymer chain, limiting the variety of functional groups that can be introduced.
In a significant advancement, a team led by Professor Shinsuke Inagi from the Department of Chemical Science and Engineering, School of Materials and Chemical Technology at Institute of Science Tokyo (Science Tokyo), Japan, has developed a postfunctionalization technique that allows for the incorporation of phosphonate esters under visible light conditions. This breakthrough paves the way for a broader range of polymer modifications.
The study, online in the journal Angewandte Chemie International Edition on May 15, 2025, is a collaborative effort involving Inagi and former graduate student Mr. Tomohiro Tamano from Science Tokyo, in partnership with Professor Hirohisa Ohmiya from Kyoto University.
The reaction is based on radical–polar crossover (RPC) chemistry, in which a carbocation is generated on the polymer backbone, enabling reactions with various nucleophiles. "Our strategy is the first example of postfunctionalization using an organophotoredox-catalyzed RPC process, significantly expanding the scope of reactions and enabling the creation of novel polymer architectures that are unattainable by other methods," says Inagi.
The process involves the phosphonylation of poly(methacrylate) derivatives containing a phthalimide ester group, utilizing the organophotoredox catalyst 12-phenyl-12H-benzo[b]phenothiazine (Ph-benzoPTZ). The proposed reaction mechanism begins with the formation of an electron donor–acceptor (EDA) complex between the phthalimide ester and the catalyst.
Upon irradiation with blue LED light, the catalyst donates an electron to the ester, causing the phthalimide group to break off along with carbon dioxide, which generates a carbon-centered radical on the polymer chain. This radical then undergoes further electron transfer or coupling with the radical cation of the catalyst, forming a carbocation equivalent (a positively charged intermediate) on the polymer chain.
Finally, this intermediate reacts with trialkyl phosphites (acting as nucleophiles), resulting in the incorporation of phosphonate groups into the polymer chain.
The resulting polymer, comprising diethyl isopropenylphosphonate, propylene, and methyl acrylate units, features a unique composition that is challenging to achieve using standard radical polymerization techniques. The team also successfully incorporated trialkyl phosphites into a precursor polymer composed of phthalimide monomers and styrene. They also created novel polymers with degrees of functionalization ranging from 7% to 21%, using various trialkyl phosphites, including chloro- and trifluoromethyl-substituted variants, demonstrating the method's broad scope and flexibility.
"Copolymerizing olefins with activated vinyl monomers is challenging and often results in low olefin incorporation, even under harsh radical polymerization conditions. However, our post-functionalization strategy enables the introduction of phosphonate groups into olefin–methacrylate copolymers, facilitating the development of unique and useful polymer structures," Inagi explains.
Polymers with phosphonate ester groups exhibit fire resistance and temperature responsiveness, even at low contents of 10%–20%. Consequently, this proposed post-functionalization strategy could be beneficial for developing flame-retardant materials and additives for lithium-ion batteries, helping to prevent battery fires. The team is now aiming to apply this strategy to incorporate other useful chemical groups into polymers for a sustainable path toward the development of next-generation functional materials.
More information: Tomohiro Tamano et al, Organophotoredox‐Catalyzed Postfunctionalization of Poly(methacrylate) Derivatives via Radical–Polar Crossover Phosphonylation, Angewandte Chemie International Edition (2025).
Journal information: Angewandte Chemie International Edition
Provided by Institute of Science Tokyo