Slowing RNA drug transport inside cells can boost effectiveness for genetic diseases

Gaby Clark
scientific editor

Robert Egan
associate editor

A recent study involving researchers from the University of Basel reveals that slowing down the intracellular transport of RNA-based drugs can significantly enhance their effectiveness. These promising therapeutics are currently used to treat rare genetic diseases.
In modern medicine, personalized therapies are becoming increasingly important—particularly in the treatment of genetic diseases. One such promising approach is the use of so-called antisense oligonucleotides (ASOs). These small, synthetic molecules specifically interfere with cell metabolism by preventing the production of disease-causing proteins. Such RNA-based therapies are already being used successfully to treat previously incurable genetic disorders such as amyotrophic lateral sclerosis (ALS) and Duchenne muscular dystrophy.
Limited efficacy of RNA-based drugs
A key challenge, however, is that most ASOs fail to reach their intended target within the cell and thus cannot achieve their full therapeutic potential. In a collaborative study in Nature Communications, an international research team—including Professor Anne Spang from the Biozentrum of the University of Basel and scientists from Roche—used CRISPR/Cas9 technology to identify factors that significantly influence ASO activity. The findings open new avenues for improving RNA therapy efficacy and accelerating their development.
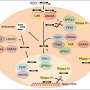
Antisense nucleotides are tiny, custom-designed genetic fragments that bind specifically to RNA molecules within the cell, thereby interfering with protein synthesis. Once administered, most ASOs are taken up by the cell and reach the cell's sorting stations, so-called endosomes, via small transport vesicles.
To exert their therapeutic effect, they must escape from the endosomes. Otherwise, they are declared as "cellular waste" and quickly shuttled to lysosomes for degradation. Since only a small fraction of ASOs manage to escape, their overall efficacy is limited.

Residence time in endosomes as a critical factor
The likelihood of ASOs escaping from the endosomes is closely linked to the speed of intracellular transport: the longer they remain in the endosome, the more time they have to escape. Using a genome-wide CRISPR/Cas9 screen, the researchers systematically knocked out thousands of genes to investigate their impact on ASO efficacy.
"We identified a large number of genes that either improve or impair ASO activity," says Dr. Liza Malong, lead author and researcher at Roche. "Many of these genes are involved in the intracellular transport of ASOs."
The team also discovered that the gene AP1M1 plays a key role in this process: it regulates the transport from the endosome to the lysosome. "By selectively switching off this gene, ASOs remain longer in specific endosomes," explains senior co-author Dr. Filip Roudnicky, also a researcher at Roche.
"This prolonged residence time increases their chance of escaping from the endosomes and becoming effective." In both cell cultures and a mouse model, this approach significantly improved ASO efficacy without requiring an increased dosage.
Toward more effective RNA-based therapies
The study provides a comprehensive overview of genes that modulate ASO activity and demonstrates that slowing down endosomal transport can boost the therapeutic efficacy of ASOs. "The key to more effective therapies thus lies not only in the drug itself, but also in intracellular trafficking," adds Spang.
"This concept may also apply to other drugs and even to bacterial and viral pathogens. Shortening the residence time of pathogens in endosomes could reduce their chance of escaping and replicating within the cell. This might represent a novel strategy in the fight against infections."
More information: Liza Malong et al, A CRISPR/Cas9 screen reveals proteins at the endosome-Golgi interface that modulate cellular anti-sense oligonucleotide activity, Nature Communications (2025).
Journal information: Nature Communications
Provided by University of Basel