Scientists visualize key protein structures linked to immune response and inflammation

Andrew Zinin
lead editor

Two key protein structures in the body are being visualized for the first time, thanks in part to the latest technology in the University of Cincinnati's Center for Advanced Structural Biology—potentially opening the door for better designed therapeutics.
The research of a trio of UC structural biologists was .
It's the first publication to come out of the Seegar Lab at UC. Tom Seegar, Ph.D., Ohio Eminent Scholar and assistant professor in the Department of Molecular and Cellular Biosciences in the College of Medicine, serves as corresponding author of the study.
The study's first authors are Joe Maciag, Ph.D., a research scientist in the Seegar Lab, and Conner Slone, a graduate student assistant in the Seegar Lab.
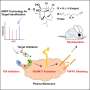
They are "seeing" the physical, atomic structure of two protein structures for the first time, along with how they interact, which can alter their function, and how that drives inflammatory signaling.
"If you can see something, you can figure out how it works," said Seegar. "We are figuring out what this enzyme looks like and how it's regulated."
What they saw
The Seegar Lab used advanced cryogenic electron microscopy techniques to visualize the structure of the ADAM17 enzyme bound to its regulator protein, iRhom2, and identify key elements vital for its activity. They were also able to highlight the essential interaction in the ADAM17-iRhom2 Complex that controls the processing of protein substrates.
Researchers know ADAM17 is a crucial enzyme present in every cell, necessary for proper development and immune regulation. They also know it becomes dysregulated in the immune systems of people with chronic inflammatory diseases and is linked to rheumatoid arthritis, cancer and COVID-19.
Now researchers are looking at the signals that ADAM17 sends to other proteins involved in immune defense and tissue repair. "We know in some cancers and rheumatoid arthritis, way too much signaling is occurring," said Maciag. "But some treatments create too many side effects, worse than the disease itself."
Maciag said researchers are now looking at how they can target iRhom2 for more selective therapeutic interventions. They have already been able to identify intracellular structural elements, dubbed the "re-entry loop" of iRhom2, that transmit information from the inside of a cell to the outside. They are required for the ADAM17 enzyme to function outside of a cell. Until now, these details have not been well understood.
Seegar emphasized the human impact. "This work provides a foundation for designing therapies targeting ADAM17-related diseases, offering new strategies to address critical health conditions," he said.
New research core facility
The ADAM17-iRhom2 Complex contains the first protein structures coming out of UC's Center for Advanced Structural Biology, established in 2022.
"We are indebted to UC," said Seegar. "Our work wouldn't be possible without this research core facility."
The cryogenic electron microscopy technology being used has transformed structural biology. The center's focal point is its transmission electron microscope (TEM), which is ideally suited for screening cryo-EM samples and allows researchers to see complex proteins without leaving UC's campus.
"It's a privilege to have this microscope in house, and it's exciting to use it to solve these structures," said Maciag. "It's important to our field of cellular biology and will help drive research forward and how we approach our understanding of inflammation in known disease states."
The Seegar Lab used UC's Advanced Research Computing Center (ARC) for data processing, allowing them to keep their data, used to create a 3D model of the ADAM17-iRhom2 Complex, on campus as well.
Moving forward, Seegar's lab plans to research iRhom2 more closely.
"These adapter proteins are not well understood," said Slone. "Our research will be in understanding them and will be driven by specificity. Ideally, controlling these will allow researchers to control disease states."
Other contributors to this study from UC: Igal Ifergan, Ph.D., assistant professor in the Department of Molecular and Cellular Biosciences in the College of Medicine; Hala Alnajjar, graduate student assistant in the Seegar Lab; Maria Rich, Medical Scientist Training Program (MTSP) student in the Seegar Lab; and Bryce Guion, an undergraduate student in the Seegar Lab.
More information: Joseph J. Maciag et al, Structural insights into the activation and inhibition of the ADAM17–iRhom2 complex, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Cincinnati