Researchers identify tuberculosis drug-resistance inhibitor and map complete structure of key bacterial pump

Gaby Clark
scientific editor

Robert Egan
associate editor

艑t膩kou Whakaihu Waka researchers have been part of two studies in the battle against drug resistant strains of Mycobacterium tuberculosis, the cause of tuberculosis.
Co-author on both studies, Dr. Matthew McNeil, of the Department of Microbiology and Immunology, describes tuberculosis as a "massive public health problem" that is challenging to solve.
"A large amount of financial investment across many years of research and development has produced some really exciting antibiotics that have now entered the clinic and are having a positive impact for affected populations.
"However, resistance to these new antibiotics has quickly emerged, and in some cases, strains are resistant even before they are exposed to the antibiotic," he says.
This antibiotic resistance can occur through many routes, one of which is efflux pumps.
"These are essentially little pumps that sit in the cell membrane and pump molecules from inside the cell to outside the cell.
"Because antibiotics look like the molecules they normally export, the cell decides to make more efflux pumps and therefore, become antibiotic resistant."
Dr. McNeil's lab is trying to both identify and understand how novel inhibitors can switch efflux pumps off in the bacteria Mycobacterium tuberculosis.
"These efflux inhibitors could be used in combination with antibiotics, enabling the antibiotic to concentrate within the cell becoming both more effective and able to stop resistance from emerging," he says.
The first study, a collaboration with researchers at the University of Cambridge and in Proceedings of the National Academy of Sciences, identified an inhibitor that is able to target and turn off a particular mycobacterial efflux pump.
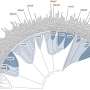
The second study, a collaboration with researchers at Shanghai Technical University and in Nature Communications, then deciphered the first complete structure of this efflux pump.

"So, we now have a scientific platform to try and understand how this new inhibitor works to stop the efflux pump and also make better versions of the inhibitor that are more potent and safer to use," he explains.
Dr. McNeil says the research is a great example of what can be achieved through collaboration.
"While establishing these scientific networks takes time and investment, by working together as part of collaborative networks of many groups with diverse expertise we can find really exciting solutions to some of the world's biggest problems."
More information: Adam J. Fountain et al, Verapamil and its metabolite norverapamil inhibit the Mycobacterium tuberculosis MmpS5L5 efflux pump to increase bedaquiline activity, Proceedings of the National Academy of Sciences (2025).
Zhiqi Xiong et al, Structure and assembly of the MmpL5/MmpS5 efflux transporter from Mycobacterium tuberculosis, Nature Communications (2025).
Journal information: Proceedings of the National Academy of Sciences , Nature Communications
Provided by University of Otago