Transforming carbon dioxide into industrial catalyst material

Gaby Clark
scientific editor

Andrew Zinin
lead editor

A research team has successfully developed a technology that efficiently converts carbon dioxide (CO2) into carbon monoxide (CO), an industrial raw material, by precisely controlling the interaction between the rhodium (Rh) catalyst and the carrier. The study is in the journal ACS Catalysis.
The team was led by Kyoungsoo Park from the Department of Â鶹ÒùÔºics and Chemistry, Daegu Gyeongbuk Institute of Science & Technology. The study was conducted in international collaboration with Professor Sungkeun Kim from Seoul National University and Professor Graham Hutchings from Cardiff University, the United Kingdom. It is expected to contribute to transforming carbon dioxide, a major cause of global warming, into a useful chemical fuel.
As carbon dioxide emissions continue to rise because of the use of fossil fuels, carbon dioxide is noted as one of the biggest contributors to global warming. Many studies have recently been conducted to convert carbon dioxide into "useful materials" instead of simply reducing its emissions.
More importantly, the "hydrogenation reaction," which enables carbon dioxide to react with hydrogen (H2) and converts it into a new material, has drawn attention as one of the critical technologies to achieve carbon neutrality. Conventional catalyst technologies, however, are underutilized, as methane (CH4) is produced as an unwanted byproduct.
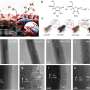
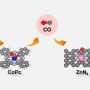
In this regard, the research team used a zinc (Zn)-based carrier (ZnO, ZnTiO3) to form a thin film called an "overlayer" on the surface of the rhodium catalyst, and they succeeded in selectively converting carbon dioxide into carbon monoxide based on this structure.
What is notable about this study is that it enabled a reaction at lower temperatures than previously possible, and the production rate of carbon monoxide was significantly increased. Carbon monoxide can be used as a key intermediate across a variety of industrial processes, including methanol, synthetic fuels, and plastic raw materials.
Furthermore, by using high-performance electron microscopy (iDPC-STEM, STEM-EELS) and real-time gas analysis techniques, the research team traced the relationship between the catalyst surface structure and the CO production pathway at the atomic level.
The team revealed the mechanism regarding "which structure generates which product," and it may serve as an underlying technology to increase precision and predictability in future catalyst designs. This technology is also applicable to carbon-neutral chemical processes under high temperature and pressure conditions, including the Fischer-Tropsch synthesis and water–gas shift reaction.
"This study holds significance as it can go beyond reducing carbon dioxide and convert it into the exact material you want," said Professor Park. "This selective catalyst design technology can be directly used in actual industries, including fuel, chemical materials, and methanol production, and it may serve as a key basis for a variety of carbon-neutral processes in the future."
More information: Rena Oh et al, Electronic and Compositional Modulation of SMSI States for Selective CO2 Hydrogenation with Rhodium Catalysts, ACS Catalysis (2025).
Journal information: ACS Catalysis