Researchers find 12 compounds that inhibit tuberculosis bacterium's energy source

Andrew Zinin
lead editor
A new study by University of Bath scientists has highlighted two new potential families of drug molecules that could open the door to new treatments for tuberculosis.
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, is the second largest infectious killer globally after COVID-19 and causes 1.3 million deaths annually.
It is the leading cause of death in people who are HIV positive, and disproportionately affects low-income populations.
While vaccination and treatments are available, treatment regimens are complicated and multi-drug resistant TB is on the rise.
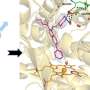
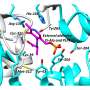
The research team, from the University's Department of Life Sciences, studied an enzyme from the TB bacterium called alpha-methylacyl-CoA racemase (MCR) which helps the bacterium use cholesterol as an energy source. Blocking this enzyme would therefore starve the bacterium of a major food source, helping to treat TB infections.
They identified 12 different chemical compounds from two chemical families that bind to MCR and inhibit its function.
They also obtained high-resolution 3D structures of MCR with and without these compounds, using X-ray crystallography techniques. The structures show how these chemicals bind to the enzyme, giving new insights into how MCR works, which the team discovered is different to the mechanism previously proposed.
These new insights will enable the team to design compounds that bind more strongly, which could eventually be used to augment the current treatments available for tuberculosis.
The paper is published in .
Dr. Matthew Lloyd, Senior Lecturer from the University of Bath's Department of Life Sciences, said, "These developments are very exciting, because for the first time we understand how the different compounds bind and have measured their strength of interaction with MCR.
"This is a very important step forward in our understanding of how this enzyme works and how its function can be blocked, potentially enabling development of better treatments for common and difficult to treat diseases."
Professor Ravi Acharya, who led the structural biology work at Bath, said, "These results give us a great handle on which molecules we should explore further for drug development.
"Next we'd like to screen large numbers of similar molecules to identify better inhibitors that could be developed into drugs."
The findings could also help drug discovery for other illnesses, since it sheds light on the function of the equivalent human enzyme, called alpha-methylacyl-CoA racemase (AMACR). The human protein is an exciting new target for the treatment of prostate cancer and other cancers.
However, progress in developing new treatments has been limited because the structure of the human protein has not yet been solved, and therefore it is difficult to fully exploit any discoveries which have been made.
While the structure of human AMACR still remains elusive, this latest paper increases understanding of the function of the human protein, potentially enabling new treatments for these cancers to be developed.
This work has been performed as part of the Ph.D. funding received from the Department of Tertiary Education & Financing (DTEF), Government of Botswana, and is the latest in a series of papers stemming from a collaboration between Acharya and Lloyd going back over 20 years.
The team plans to extend this work to investigate how other known compounds bind to the protein, to further understand how the structure of the compound influences its binding.
More information: Otsile O. Mojanaga et al, Molecular basis of acyl-CoA ester recognition by α-methylacyl-CoA racemase from Mycobacterium tuberculosis, Journal of Biological Chemistry (2025).
Journal information: Journal of Biological Chemistry
Provided by University of Bath