Destabilizing microtubules to boost platelet production from iPS cell-derived megakaryocytes

Lisa Lock
scientific editor

Robert Egan
associate editor

A collaborative research team led by Dr. Thorsten Schlaeger (Boston Children's Hospital) Emiri Nakamura and Professor Koji Eto (Department of Clinical Application) has identified microtubule (MT) destabilization as a promising strategy to enhance platelet production from human iPS cell-derived immortalized megakaryocyte progenitor cell lines (imMKCLs). The work is in PLOS One.
Platelet transfusions are essential for managing thrombocytopenia and bleeding disorders, yet donor-derived platelets face limitations such as short shelf life, supply shortages, and immune incompatibility.
To address these critical issues, the Eto Lab has developed imMKCLs—conditionally immortalized megakaryocyte lines derived from human iPS cells—which can be expanded and matured to produce functional platelets. However, platelet yields remain suboptimal under static culture conditions.
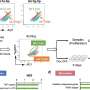
Using single-cell time-lapse imaging, the research team observed that imMKCLs exhibit asynchronous and heterogeneous platelet production, with only a minority of cells releasing large numbers of platelet-like particles (PLPs). A high-content chemical genetics screen identified MT-destabilizing agents, particularly vinca alkaloids such as vincristine (VCR), as potent enhancers of proplatelet extension formation.
VCR treatment on day three of differentiation significantly increased PLP output, especially under turbulence-enhanced culture conditions using the VerMES bioreactor. These findings demonstrate that timed microtubule destabilization enhances platelet biogenesis from iPS cell-derived megakaryocytes without compromising function.
Further analysis revealed an inverse correlation between MT content and platelet yield. Compared to static cultures, imMKCLs cultured under turbulent flow showed reduced MT staining and higher platelet productivity. VCR addition further boosted yields, with optimal effects observed when applied on day 3, after polyploidization but before proplatelet formation. Early or late VCR treatment diminished efficacy, highlighting a critical time-window for intervention.
Importantly, platelets produced under VCR treatment retained key functional properties. Flow cytometry and confocal imaging confirmed responsiveness to activation agonists, low Annexin V binding, and the ability to spread on fibrinogen-coated surfaces. In vivo experiments using thrombocytopenic NSGS-SGM3 mice demonstrated that VCR-treated iPS cell-derived platelets restored hemostasis effectively, although higher VCR doses slightly reduced platelet persistence and marginal band structure.
This study underscores the role of MT dynamics in megakaryocyte maturation and platelet biogenesis. By leveraging FDA-approved compounds like VCR, the researchers present a practical method to enhance platelet yields from iPS cell-derived sources. These insights pave the way for scalable, cost-effective platelet production systems for transfusion medicine, with future work aimed at elucidating the underlying mechanism of MT modulation and its role during maturation.
More information: Emiri Nakamura et al, Association of microtubule destabilization with platelet yields in terminally differentiating hiPSC-derived megakaryocyte lines, PLOS One (2025).
Journal information: PLoS ONE
Provided by Kyoto University