Research on ice-forming compound could improve pipeline safety, carbon capture and storage

Lisa Lock
scientific editor

Robert Egan
associate editor

Canadians may think they're intimately familiar with ice in all its forms, but there is one kind that most have probably never heard of. Clathrate hydrates are tiny crystalline cages of ice that can trap other gases or liquids inside them.
These hydrates can form in natural gas pipelines and cause explosions if they block the line. The BP Deepwater Horizon disaster in the Gulf of Mexico in 2010 was caused by hydrate formation, says John Tse, Canada Research Chair of Materials Science and a professor in the Department of Â鶹ÒùÔºics and Engineering Â鶹ÒùÔºics at the University of Saskatchewan (USask).
That's one of the reasons Tse and his colleagues "want to understand more about how this compound forms, and how the gas and water interact with each other."
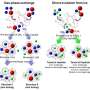
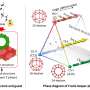
Because the reactions that form hydrates happen so quickly, the researchers needed a way to both slow them down and observe them in progress. So Tse cooled down a mixture of water and a chemical called tetrahydrofuran (THF) to -263°C in a vacuum, then used the powerful X-ray beamlines of the Canadian Light Source at USask to watch how the molecules moved and changed shape as he slowly warmed up the mixture.
Tse found that, as the temperature rose, the THF separated out and formed crystals while the frozen water remained in a non-crystal form. Then, around -163°C, the THF suddenly melted and mixed with the water to form clathrate hydrates, crystalline cages of ice with THF trapped inside. The work is in The Journal of Â鶹ÒùÔºical Chemistry Letters.
Understanding more about how hydrates behave could lead to many different practical applications beyond just protecting against pipeline explosions. They could also be used in natural gas transport and storage—a single cubic foot of hydrate can store up to 150 cubic feet of gas—or for carbon capture and storage projects. Tse hopes that his fundamental science work will be used by more applications-minded engineers to develop helpful new technologies.
"Everything in science is baby steps," says Tse. "We start with idealistic conditions and go toward practical ends. Someone might pick this up with a smart idea that relies on fundamental knowledge about how hydrates are formed."
More information: Robert P. C. Bauer et al, Capturing Hydrate Formation Processes in Tetrahydrofuran/Water Mixtures with Temperature Resolved In Situ Synchrotron X-ray Diffraction and Infrared Spectroscopy, The Journal of Â鶹ÒùÔºical Chemistry Letters (2025).
Journal information: Journal of Â鶹ÒùÔºical Chemistry Letters
Provided by Canadian Light Source