Organic molecules show promise for sensitive quantum sensing through color-shifting spins

Sadie Harley
scientific editor

Andrew Zinin
lead editor

Quantum sensing has transformational potential across many areas of technology and science, most prominently biomedical research. The basic premise is to detect and manipulate the spin state of an electron—magnetic properties of electrons that can be used to store quantum information using light. This capability has previously been limited to highly exotic or expensive materials such as nano-sized diamonds with specific atomic defects.
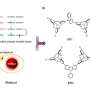
Now, in a paper published in , scientists have reported an organic molecule built from carbon atoms in which its optical properties are intrinsically linked to its electron's spin. It's based on two small molecular units, each carrying an unpaired electron (known as spin radical). \
When these two units are connected to form a diradical, the two electron spins can align in two different ways: pointing in the same direction (called a triplet state) or in opposite directions (a singlet state).
"The fine-tuned molecular design is key to achieving reliable interaction between the two spin radical units," says Dr. Petri Murto, working in the group of Professor Hugo Bronstein, Yusuf Hamied Department of Chemistry, University of Cambridge.
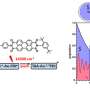
The interaction between the two electron spin configurations controls the color of the molecule when a photon—a particle of light—is absorbed in the diradical.
"When the two electron spins are pointing in the same direction, the molecule emits orange light; but when the two electron spins are pointing in opposite directions, the molecule emits near infra-red light," explains Rituparno Chowdhury, first author and Ph.D. student working in the group of Professor Sir Richard Friend in the Department of Â鶹ÒùÔºics, University of Cambridge.
"This allows you to very easily detect and know the quantum states of the molecule just by looking at the color."
Because the quantum states of a molecule are extremely sensitive to their environment—such as magnetic fields, temperature, or chemical surroundings—scientists can detect changes in the environment with far greater sensitivity than using traditional ('classical') materials.
The color shift observed is connected to a known model for magnetic materials where the Hubbard energy is the cost of placing two electrons on the same site. This model is widely used for inorganic materials, including high-temperature superconductors.
"By applying a magnetic field, we can push the molecule into the triplet state and make it glow orange. At low temperatures, without the field, the singlet state dominates, and the molecule glows in the near infrared. With microwave pulses, they can also drive transitions between the states—a kind of coherent spin control normally seen in much more complex solid-state systems," adds Dr. Alexei Chepelianskii, Université Paris-Saclay.
"The color output can be tuned using temperature or a magnetic field. I would never have believed materials like this could even exist. This opens up a whole new class of carbon-based materials with controllable spin-optical properties—materials that are not only highly luminescent but also much simpler to process than traditional materials," notes Professor Hugo Bronstein, Yusuf Hamied Department of Chemistry, University of Cambridge.
In an earlier research, scientists at the Cavendish Laboratory had already shown that individual spin-radical units could be used to make highly efficient organic light-emitting diodes (OLEDs) operating in the red and near-infrared.
"With this new advance, we have taken a step further: showing how spin interactions in carefully designed diradical molecules can tune how the molecule responds to light, and in turn, how that light can be used to read out or even control the spin state," remarks Professor Sir Richard Friend, Cavendish Laboratory, University of Cambridge.
This new discovery opens the door to molecular-based quantum information and sensing technology, where small size, chemical control and low cost could accelerate implementation.
More information: Chowdhury, R., et al. Bright triplet and bright charge-separated singlet excitons in organic diradicals enable optical read-out and writing of spin states. Nature Chemistry (2025).
Journal information: Nature Chemistry
Provided by University of Cambridge