Curved molecules store sunlight as chemical energy and release heat on demand

Sanjukta Mondal
contributing writer

Lisa Lock
scientific editor

Andrew Zinin
lead editor

Curved molecules that absorb sunlight, store the energy, and re-release it as heat are pushing the boundaries in solar thermal storage technology.
In a recent study in the journal Chem, researchers revealed curved anthracene derivatives—organic molecules found in coal tar—that undergo structural changes upon absorbing sunlight and, when triggered to return to their original shape, release the stored solar energy as heat.
The anthracene systems, derived from a by-product of the fossil fuel industry, exhibited high energy storage densities, making them promising solvent-free alternatives to traditional thermal energy storage systems.
Molecular solar thermal (or MOST) energy storage works on the principle of storing energy in chemical bonds. It relies on specially designed photosensitive molecules that undergo reversible structural change known as Dewar isomerization. When exposed to light, the molecules shift from a stable form to a high-energy form, and in the process, they trap solar energy in strained chemical structures.
MOST material can keep the energy locked in for long periods of time and the energy can be released if and when required by simply triggering the system with either heat, light of a specific wavelength or a catalyst.

Sunlight is a cocktail of light with different wavelengths, and existing MOST systems miss harnessing a major portion of it as they tend to only absorb ultraviolet (UV) light, leaving visible light untapped. They also require dilute organic solvents to operate, which leads to low gravimetric energy densities (energy stored per unit mass).
These new curved anthracene systems, designed by the researchers, not only absorb visible light but are also solvent-free, and experienced more than 28 cycles with minimal fatigue.
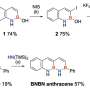
The researchers chose anthracene—an organic molecule made up of three benzene rings fused in a linear arrangement—as the starting molecule. They designed four distinct derivatives by introducing different bulky groups at the 9-position of the anthracene to ensure that the molecule attains a bent structure, as regular anthracene tends to form a dimer upon absorbing energy rather than forming an isomer. The formation of isomers is crucial to the process of energy storage and subsequent release.

The designed systems exhibited efficient Dewar isomerization upon visible-light absorption, achieving high energy storage densities of up to 170 kJ/mol and 0.65 MJ/kg, values comparable to those of popular materials on the market. The anthracene systems, which were in the liquid phase, required no solvent for operation, which maximized the usable energy.
One of the systems underwent 28 cycles of photo-induced Dewar isomerization and thermally triggered reversion with minimal decline in absorbance values and no significant degradation. All four systems were able to recharge themselves under simulated sunlight within 6–8 hours, ready to release thermal energy when required.
The researchers note that this study demonstrated the potential of fully carbon-based systems to store substantial amounts of solar energy, marking a significant step toward practical and scalable solar thermal energy storage.
Written for you by our author , edited by , and fact-checked and reviewed by —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Subhayan Chakraborty et al, Curved anthracenes for visible-light photon energy storage via Dewar isomerization, Chem (2025).
Journal information: Chem
© 2025 Science X Network