Study reveals how Pd and Pt catalyst surfaces control chemical production

Lisa Lock
scientific editor

Andrew Zinin
lead editor

Using palladium (Pd) or platinum (Pt) catalysts for propylene electrooxidation offers a sustainable route to producing valuable oxygen-containing organic compounds used in various industrial processes. However, the reasons why these catalysts produce different products depending on the applied potential have remained unclear.
A research group has offered greater clarity with a new study recently in the Journal of the American Chemical Society. They combined several advanced theoretical techniques—grand-canonical ensemble density functional theory (DFT) calculations, Pourbaix analyses, and microkinetic modeling—to map out the full reaction network of propylene electrooxidation. Their findings reveal that changes to the surfaces of Pd and Pt electrodes under working potentials govern which products are formed.
One way to understand this is to imagine the catalyst surface as a key that changes shape depending on the voltage applied. For Pd electrodes, increasing the potential (0.7–1.4 V vs. RHE) gradually changes the surface from partially oxygen-covered metallic Pd to PdO with partial hydroxylation.
As the "key" shape changes, it unlocks different reaction pathways, shifting the main product from acrolein to acetone and propylene glycol. In contrast, the Pt electrode maintains a stable PtO2 surface with partial hydroxylation across the studied potential range (1.2–1.6 V vs. RHE), consistently producing propylene oxide and acetone.
-

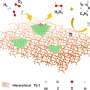
Reaction network for propylene electrooxidation. The studied intermediates and elementary steps along the pathways to PO, PG, acrolein, and acetone for propylene electrooxidation on 1/3 ML O*-covered Pd(111). Credit: Danyang Li et al -

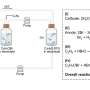
The product selectivity evolution of propylene electrooxidation as a function of applied electrode potential over Pd catalyst. (a) Pd 3d XPS spectra of Pd/C electrode recorded through ex-situ in N2-saturated H3PO4/NaH2PO4 electrolyte. Credit: Danyang Li et al
This work resolves longstanding questions about what determines the products of propylene electrooxidation on Pd and Pt catalysts. The insights can guide the design of better catalysts for sustainable chemical production by highlighting the importance of surface reconstruction and active site switching under working conditions.
The research team also utilized the Digital Catalysis Platform, developed by the Hao Li Lab, to support their analyses and modeling.
"Understanding how surface reconstruction affects reaction selectivity is like discovering how a key's shape controls which doors it can open. With this knowledge, we can design catalysts to produce target chemicals more efficiently," said Hao Li (WPI-AIMR, Tohoku University), who led the research.
More information: Danyang Li et al, Unraveling the Potential-Dependent Selectivity of Propylene Electrooxidation: The Role of Electrochemistry-Induced Reconstruction, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by Tohoku University