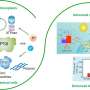
Cryo-EM maps of four distinct TEF30-PSII complexes. Credit: Nature Plants (2025). DOI: 10.1038/s41477-025-02036-3
Photosystem II (PSII) is the only biological machine capable of splitting water into oxygen using sunlight. It plays a fundamental role in global oxygen production and solar energy conversion. However, PSII is highly prone to light-induced damage, particularly under high-light conditions.
Repair of PSII requires disassembling and reassembling of its complex components, as well as replacement of the D1 protein in the reaction center. While previous studies illuminated the early phases of PSII repair, the mechanisms governing its reassembly remained largely elusive until now.
In a study in Nature Plants on June 27, a research team led by Prof. Liu Zhenfeng from the Institute of Biophysics of the Chinese Academy of Sciences has solved four high-resolution cryo-electron microscopy (cryo-EM) structures that unveil how a critical protein, Thylakoid Enriched Fraction 30 (TEF30), facilitates the repair and assembly of PSII in Chlamydomonas reinhardtii, a model green alga.
These findings offer unprecedented insights into the mid-to-late stages of PSII repair, a vital process for sustaining oxygenic photosynthesis in algae and plants.
The researchers combined antibody affinity purification with sucrose density gradient ultracentrifugation to isolate the PSII-repair intermediate complexes associated with TEF30. Using single-particle cryo-EM, they solved the near-atomic resolution structures of four TEF30-containing PSII intermediate complexes, named TEF30-C, TEF302-C2-I, TEF302-C2-II, and TEF30-C2S.
Structural analysis revealed that TEF30 binds to the stromal side of the PSII core complex (PSII-C), forming multiple polar interactions with four core subunits of PSII: D1, D2, CP43, and PsbI. Biolayer interferometry (BLI) assays demonstrated a high binding affinity between TEF30 and PSII-C.
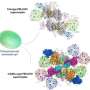
A proposed molecular model for the TEF30-mediated mechanism of the mid-to-late stage PSII repair process. Credit: Liu Zhenfeng's group
By aligning and comparing the structures of TEF302-C2-I, TEF302-C2-II, TEF30-C2S, and the mature PSII supercomplex (PSII-SC), the study, for the first time, identified several distinct intermediate forms of PSII-C dimers.
Based on biochemical data and cryo-EM structures, the researchers proposed a working model illustrating how different modules reassemble through the process mediated by TEF30 during the mid-to-late stages of the PSII repair cycle, ultimately leading to the formation of a mature PSII supercomplex.
"Understanding the roles of TEF30 in the PSII repair process is critical for advancing our knowledge of the fundamental molecular events occurring in photosynthetic organisms under constant light stress," said Prof. Liu.
"These structural insights not only provide a near-atomic framework for future investigations on photosystem II maintenance, but may also inspire endeavors in improving photosynthetic efficiency of crops."
More information: Yidi Wang et al, Roles of multiple TEF30-associated intermediate complexes in the repair and reassembly of photosystem II in Chlamydomonas reinhardtii, Nature Plants (2025).
Journal information: Nature Plants
Provided by Chinese Academy of Sciences